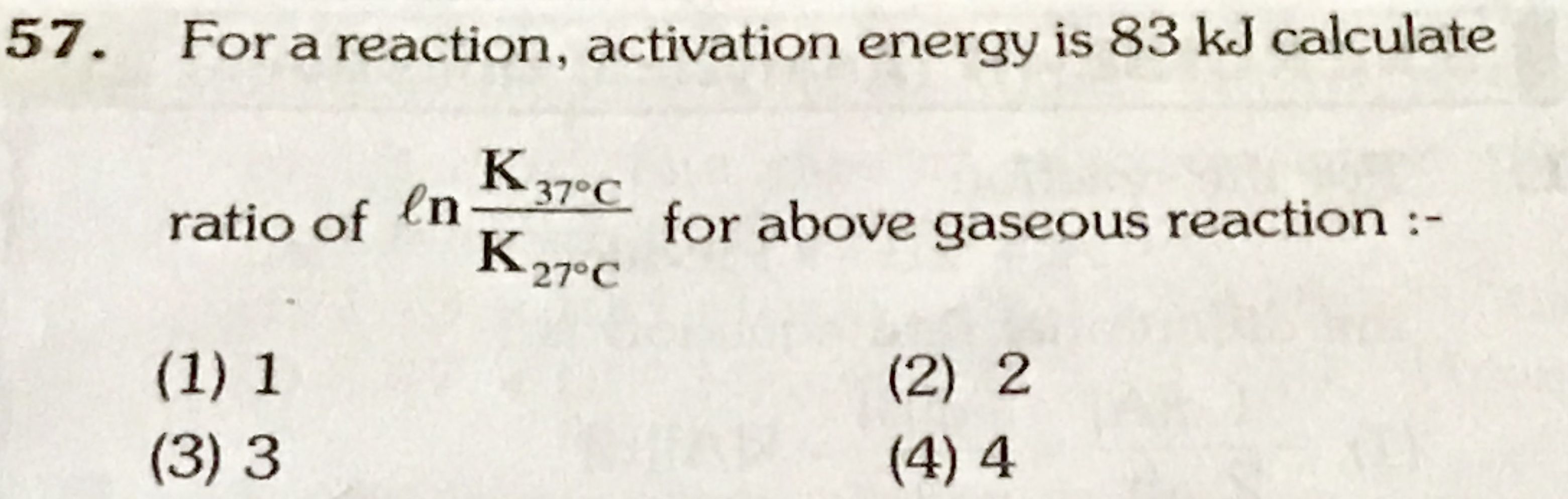Chemical Kinetics
Chemical Kinetics PDF Notes, Important Questions and Synopsis
SYNOPSIS
Rate of chemical reaction:
- The rate of chemical reaction is the change in concentration over the change in time.
Types of rates of chemical reaction:
- Average rate: The rate of reaction measured over a long time interval is called the average rate of reaction.
- Instantaneous rate: It is the rate of reaction when the average rate is taken over a very small interval of time.
- Rate = K (conc.)order − differential rate equation or rate expression,
where K = rate constant = specific reaction rate = rate of reaction when concentration is unity - unit of K = (conc)1- order time-1
- Total number of atoms, ions or molecules of the reactants involved in the reaction is termed its molecularity.
- m1A + m2B→products
- R ∝ [A]P [B]q, where p may or may not be equal to m1 and the similarly q may or may not be equal to m2.
- p is the order of reaction with respect to reactant A, q is the order of reaction with respect to reactant B and (p + q) is the overall order of the reaction.
- The rate of reaction does not change with the concentration of the reactants.
- Rate = k [conc.]° = constant
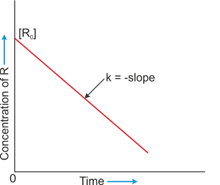
- The reaction in which the rate of reaction is directly proportional to the concentration of a reacting substance.
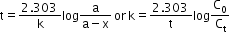
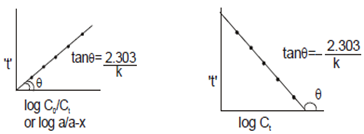
- Half-life: The time taken for a reaction when half of the starting material has reacted is called half-life of the reaction.

Second order reaction:
- The reaction in which the sum of powers of concentration terms in rate law or rate equation is two.
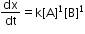
Pseudo first order reaction:
- The reaction which is bimolecular, but the order is one is called a pseudo first order reaction; for example, acidic hydrolysis of ester.
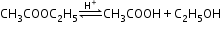
Third order reaction:
- The reaction in which the sum of the powers of concentration terms in rate law or rate equation is equal to three.
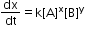 where x + y = 3
where x + y = 3
Specific rate constant (k):
- It is equal to the rate of reaction when the molar concentration of the reactant is unity.
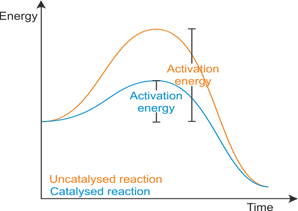
Activation energy:
- The minimum amount of energy that is required to activate atoms or molecules to start a reaction.
Initial rate:
- The rate at the beginning of the reaction when the concentrations have not changed appreciably.
- Greater the surface area, more will be the rate of reaction.
- Rate of reaction increases with the increase in concentration in general except in zero order reaction.
-
When we increase the temperature, the number of molecules possessing activation energy increases because average kinetic energy of molecules increases. So, increasing the temperature increases the reaction rates.
-
A catalyst increases the rate of reaction by lowering the activation energy.
- It is based on the kinetic theory of gases. A chemical reaction takes place as a result of reacting collisions.
- Collision frequency (Z): The number of collisions which takes place per second per volume of the reaction mixture is called collision frequency.
- Effective collision: Collisions which lead to the formation of product molecules are called effective collisions.
- The rate of reaction depends on the number of effective collisions.
- Radioactivity is the spontaneous emission of penetrating rays in the form of particles or high-energy photons resulting from a nuclear reaction.
- These penetrating rays are classified in three categories—alpha, beta, gamma.
-
Law of Radioactive Decay (Rutherford and Soddy Law):
According to this law, the activity of radioactive nuclei is directly proportional to the number of radioactive nuclei present at any instant.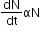
-
Law of Radioactive Displacement Law (Group Displacement Law):
On the emission of an alpha particle, the new element lies two columns left in the periodic table and the mass number decreases by 4 points.
On the emission of a beta particle, the new element lies one column right in the periodic table and the mass number remains the same. -
Radioactive Series:
Series
Parent element
End element
4n series
Th-232
Pb-208
4n+1 series
Pu-241
Bi-209
4n+2 series
U-238
Pb-206
4n+3 series
U-235
Pb-207
-
Radioactive Equilibrium:
Radioactive change is an irreversible process, but it shows equilibrium when a daughter element disintegrates at the same rate at which it is formed from parent element. -
Nuclear Fission:
The phenomenon of splitting up of a heavy nucleus on bombardment with slow speed neutrons is known as nuclear fission. -
Nuclear Fusion:
The phenomenon of joining of two lighter nuclei into a heavier nucleus is called nuclear fusion. -
Dating:
Radioactive dating means determining the age of a mineral specimen by determining the relative amounts present in certain radioactive elements.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry