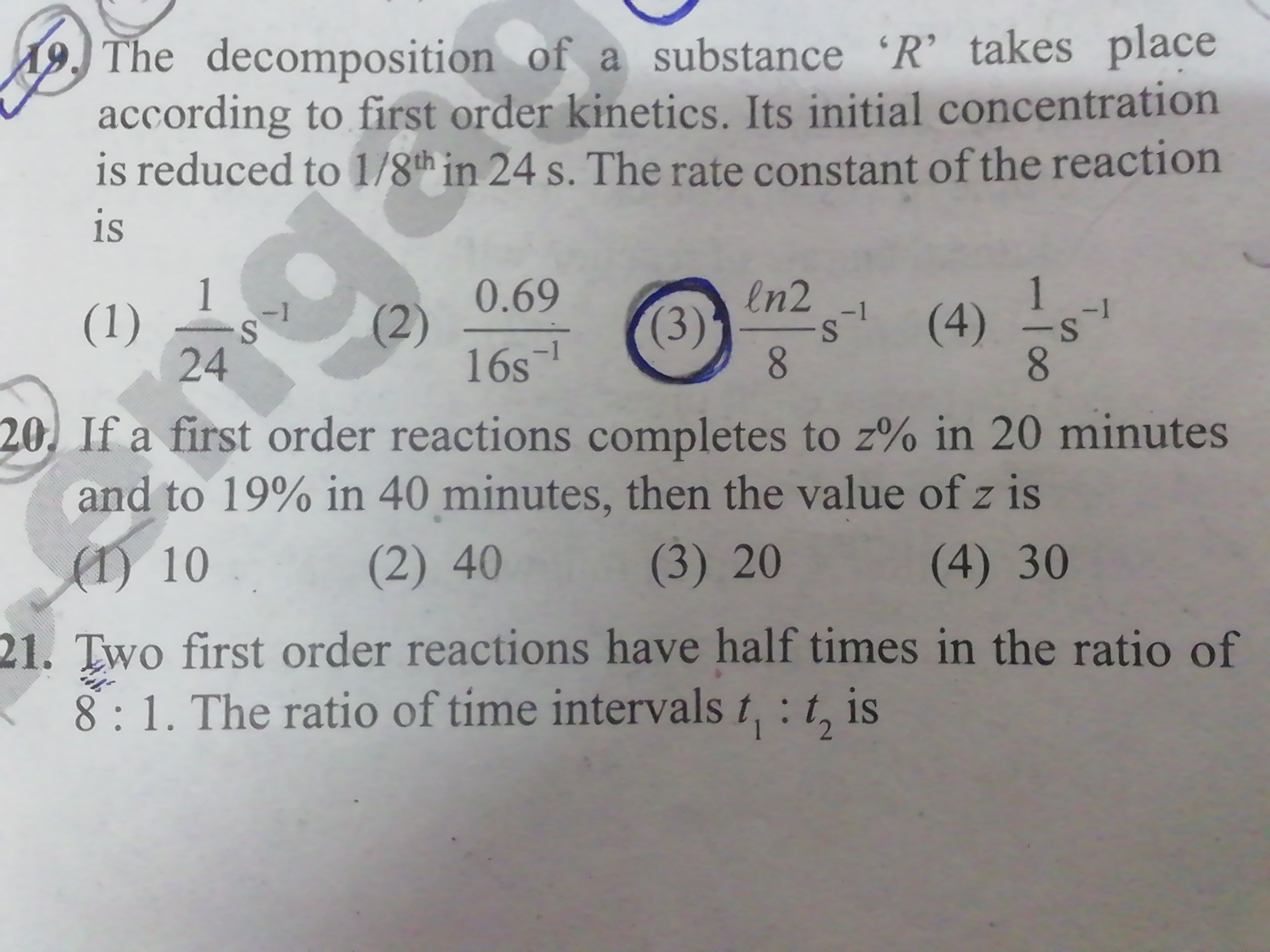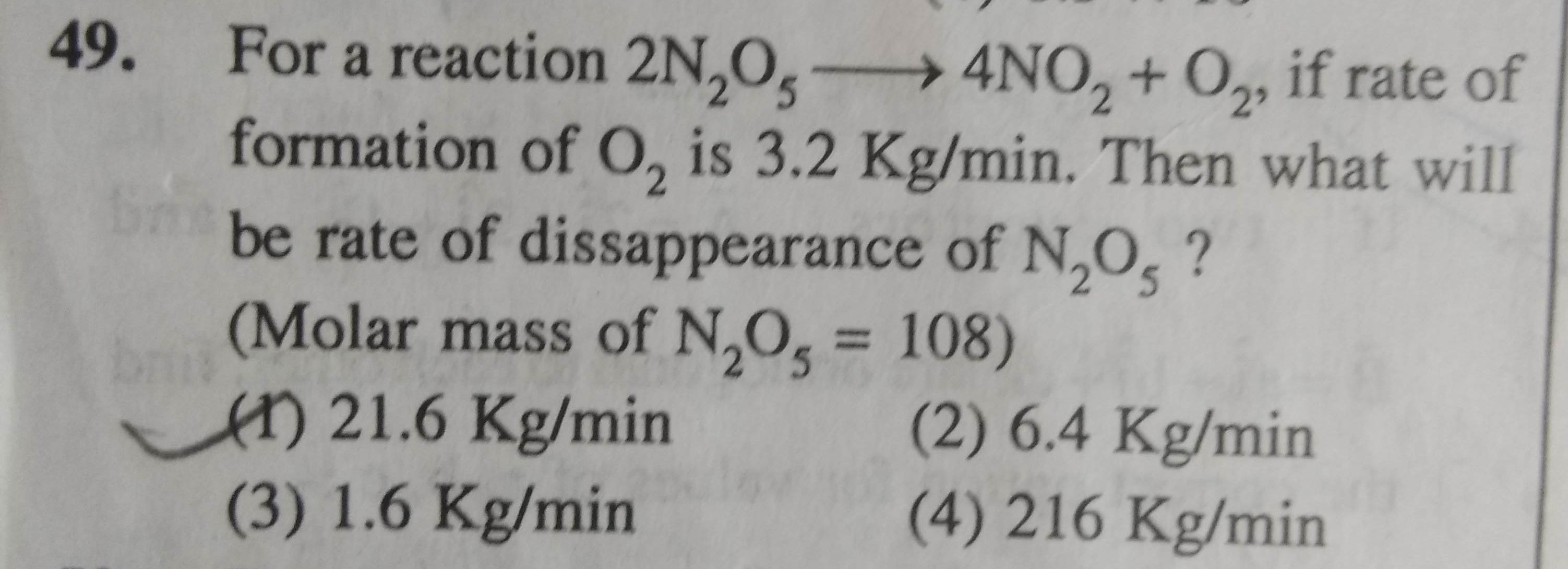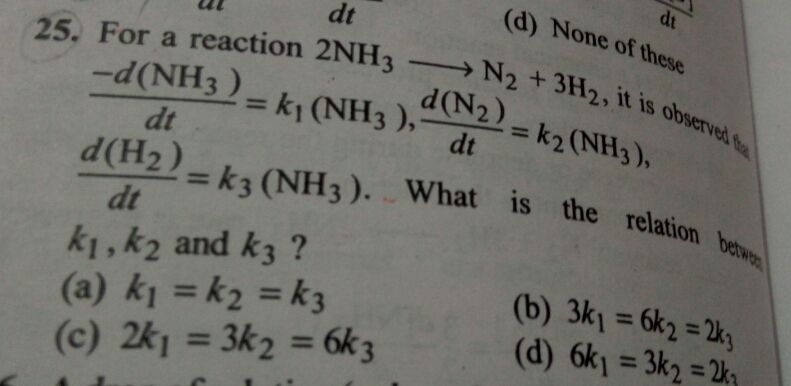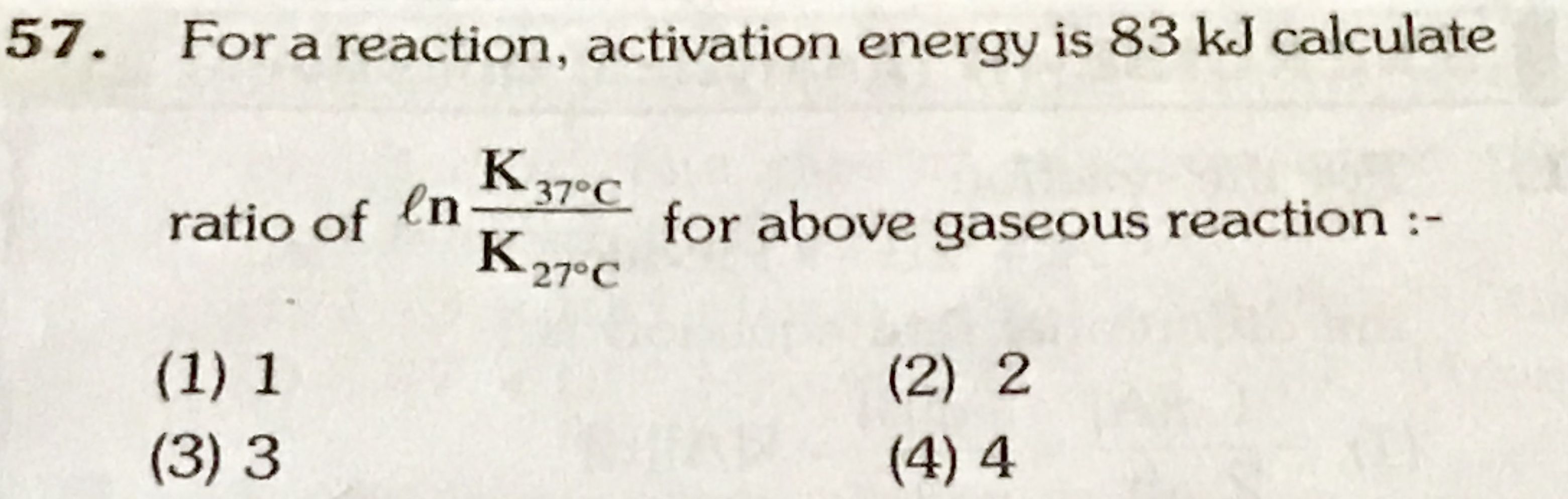NEET Class neet Answered
what will be the rate of decomposition of n2o5 and rate of formation of no2 and o2......
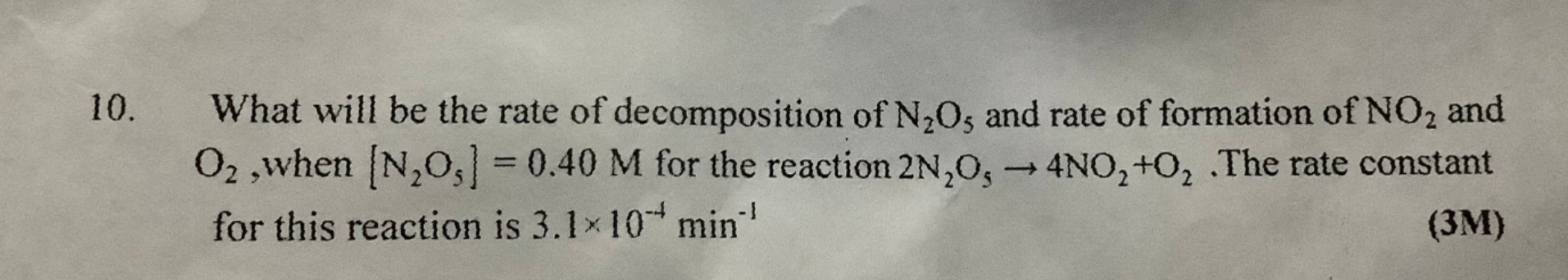
Asked by gamingwithdjokonitin | 01 May, 2022, 10:20: PM
The rate of reaction is calculated by-
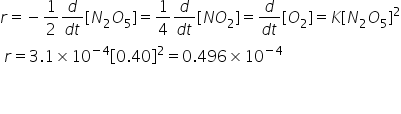
Answered by Ravi | 02 May, 2022, 11:22: PM
NEET neet - Chemistry
Asked by imamsyed65p | 12 Jul, 2022, 12:14: PM
NEET neet - Chemistry
Asked by gamingwithdjokonitin | 01 May, 2022, 10:20: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 09 Aug, 2021, 12:18: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 02 Aug, 2021, 02:04: PM
NEET neet - Chemistry
Asked by patra04011965 | 14 Mar, 2021, 07:29: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 27 Oct, 2020, 05:11: PM
NEET neet - Chemistry
Asked by jhajuhi19 | 25 Oct, 2020, 06:01: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 28 Feb, 2020, 10:02: PM
NEET neet - Chemistry
Asked by valavanvino1011 | 20 Jul, 2019, 08:35: PM
NEET neet - Chemistry
Asked by astutijoshi | 05 Jul, 2019, 06:17: PM