General Principles and Processes of Isolation of Metals
General Principles and Processes of Isolation of Metals, PDF Notes, Important Questions and Synopsis
SYNOPSIS
METALLURGY
The compound of a metal found in nature is called a mineral. The minerals from which metals can be economically and conveniently extracted are called ores. An ore is usually contaminated with earthy or undesired materials known as gangue.
- Native ores contain the metal in the free state. Silver, gold and platinum occur as native ores.
- Oxidised ores consist of oxides or oxysalts, such as carbonates, phosphates, sulphates and silicates of metals.
- Sulphurised ores consist of sulphides of metals like iron, lead, zinc and mercury.
- Halide ores consist of halides of metals.
Metal
Ore
Composition
Aluminium
Bauxite
AlOX(OH)3-2X [where 0< X<1] Al2O3
Diaspore
Al2O3.H2O
Corundum
Al2O3
Kaolinite (a form of clay)
[Al2(OH)4Si2O5]
Iron
Haematite
Fe2O3
Magnetite
Fe3O4
Siderite
FeCO3
Iron pyrite
FeS2
Limonite
Fe2O3.3H2O
Copper
Copper pyrite
CuFeS2
Cuprite
Cu2S
Malachite
CuCO3.Cu(OH)2
Azurite
2CuCO3.Cu(OH)2
Zinc
Zinc blende or sphalerite
ZnS
Calamine
ZnCO3
Zincite
ZnO
Lead
Galena
PbS
Anglesite
PbSO4
Cerussite
PbCO3
Magnesium
Carnallite
KCl.MgCl2.6H2O (K2MgCl4.6H2O)
Magnesite
MgCO3
Dolomite
MgCO3,CaCO3
Epsom salt (Epsomite)
MgSO4 7H2O
Langbeinite
K2Mg2(SO4)3
Tin
Cassiterite (Tin stone)
SnO2
Silver
Silver glance (Argentite)
Ag2S
Chlorargyrite (Horn silver)
AgCl
Metallurgy:
The scientific and technological process used for the extraction/isolation of the metal from its ore.
Isolation and extraction of metals from their ores involve the following major steps:
- Crushing and grinding: The ore is first crushed by jaw crushers and ground to a powder.
- Concentration:
- Hydraulic washing or gravity separation or levigation method
- Electromagnetic separation
- Froth flotation process
- Leaching
- Extraction of crude metal from concentrated ore:
The removal of unwanted useless impurities from the ore is called dressing, concentration or benefaction of ore.
Isolation of metals from their concentrated ores involves two major steps: - Conversion to oxide:
Calcination: Process of strongly heating the concentrated ore in a limited supply of air or in the absence of air.
Roasting: Process of strongly heating the concentrated ore (generally sulphide ore) in excess of air or O2 below its melting point.
Smelting: In many extraction processes, an oxide is added deliberately to combine with other impurities and form a stable molten phase which is immiscible with the molten metal called slag. This process is termed smelting.
Slag formation:
2CuFeS2 + 4O2 Cu2S + 2FeO + 3SO2
Cu2S + 2FeO + 3SO2 
CaCO3
 CaO+CO2
CaO+CO2
CaO + SiO2 CaSiO3 (fusible slag)
CaSiO3 (fusible slag)
6CaO + P4O10 2Ca3(PO4 )2 (fusible slag: Thomas slag)
2Ca3(PO4 )2 (fusible slag: Thomas slag) - Reduction of a metal oxide:
The free metal is obtained by reduction of a compound using either a chemical reducing agent or electrolysis.
Chemical reduction method:
Reduction with carbon:
PbO + C Pb + CO (extraction of lead)
Pb + CO (extraction of lead)
Reduction with CO:
Fe2O3 + 3CO 2Fe + 3CO2
2Fe + 3CO2
Reduction by other metals:
The process is known as Goldschmidt or aluminothermic process and the reaction is known as thermite reaction.
Cr2O3 + Al 2Cr + Al2O3
2Cr + Al2O3
Magnesium reduction method:
Self-reduction method:
This method is also called auto-reduction method or air reduction method.
Cu2S + 3O2 3Cu2O + 2SO2
3Cu2O + 2SO2
2Cu2O + Cu2S 6Cu + SO2
6Cu + SO2Electrolytic reduction:
1. In aqueous solution: Copper and zinc are obtained by electrolysis of aqueous solution of their sulphates.
2. In fused melts: Aluminum is obtained by electrolysis of a fused mixture of AI2O3 and cryolite Na3[AIF6].
Extraction of aluminium: It involves the following processes:- Purification of bauxite:
- Bayer’s method:
(used for red bauxite containing Fe2O3 and silicates as impurities)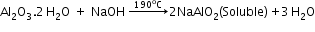
- Hall’s method:
(used for red bauxite containing Fe2O3 and silicates as impurities)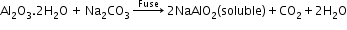
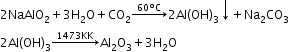
- Serpeck’s method:
(used for white bauxite containing silica as impurities)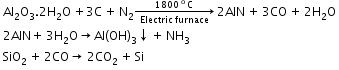
- Silicon volatilises at this temperature
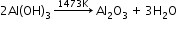
- Bayer’s method:
- Electrolytic reduction (Hall–Heroult process):
2Al2O3 + 3C 4Al + 3CO2
4Al + 3CO2
Cathode: Al3+ (melt) + 3e− Al(l)
Al(l)
Anode: C(s) + O2- (melt) CO(g) + 2e-
CO(g) + 2e-
C(s) + 2O2-(melt) CO2(g) + Ae-
CO2(g) + Ae-
Metallurgy of some important metals:
- Extraction of iron from haematite:
Reactions involved:
At 500–800 K (lower temperature range in the blast furnace):
3Fe2O3 + CO 2Fe3O4 + CO2
2Fe3O4 + CO2
Fe3O4 + CO 3Fe + 4CO2
3Fe + 4CO2
Fe2O3 + CO 2FeO + CO2
2FeO + CO2
At 900–1500 K (higher temperature range in the blast furnace):
C + CO2 2CO; FeO + CO
2CO; FeO + CO Fe + CO2
Fe + CO2
Limestone is also decomposed to CaO which removes the silicate impurity of the ore as slag. The slag is in the molten state and separates out from iron.
CaCO3 CaO + CO2; CaO + SiO2
CaO + CO2; CaO + SiO2  CaSiO2
CaSiO2 -
Extraction of copper:
From copper glance/copper pyrite (self-reduction):
2CuFeS2 + 4O2 Cu2S + 2FeO + 3SO2
Cu2S + 2FeO + 3SO2
Cu2S + FeO + SiO2 FeSiO3 (fusible slag) + Cu2S (matte)
FeSiO3 (fusible slag) + Cu2S (matte)
2FeS + 3O2 2FeO + 2SO2; FeO + SiO2
2FeO + 2SO2; FeO + SiO2  FeSiO3
FeSiO3
2Cu2O + Cu2S 6Cu + SO2 (self-reduction)
6Cu + SO2 (self-reduction) -
Extraction of lead:
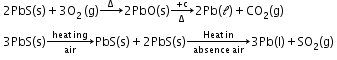
-
Extraction of zinc from zinc blende:
The ore is roasted in the presence of excess of air at a temperature of 1200 K.
2ZnS + 3O2 2ZnO + 2SO2
2ZnO + 2SO2
Zinc oxide is reduced using coke.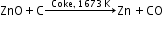
-
Extraction of tin from cassiterite:
SnO2 + 2C
 Sn + 2CO
Sn + 2CO
2Fe + O2 2FeO
2FeO -
Extraction of magnesium:
From sea water (Dow’s process):
MgCl2 ⇌ Mg2+ + 2Cl⎺
At cathode: Mg2+ + 2e⎺ Mg (99% pure)At anode: 2Cl⎺
Mg (99% pure)At anode: 2Cl⎺ Cl2 + 2e⎺
Cl2 + 2e⎺ -
Extraction of gold and silver (MacArthur–Forrest cyanide process):
- From native ores: Extraction of gold and silver involves leaching the metal with CN-.
4Au/Ag(s) + 8CN⎺(aq) + 2H2O(aq) + O2(g) 4[Au/Ag(CN)2] ⎺ (aq) + 4OH⎺ (aq)
2[Au/Ag(CN)2] ⎺ (aq) + Zn(s) 2Au/Ag(s) + [Zn(CN)4]2⎺ (aq) - From argentite ore:
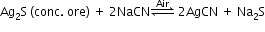
4Na2S + 5O2 + 2H2O
 2Na2SO4 + 4NaOH + 2S
2Na2SO4 + 4NaOH + 2S
AgCN + NaCN Na[Ag(CN)2] (soluble complex)
Na[Ag(CN)2] (soluble complex)
2Na[Ag(CN)2] + Zn (dust) 2Ag↓ + Na2[Zn(CN)4]
2Ag↓ + Na2[Zn(CN)4]
- Purification of bauxite:
-
Purification or refining of metals:
Physical methods:
- Liquation process: This process is used for the purification of the metal, which itself is readily fusible, but the impurities present in it are not. It is used for the purification of Sn and Zn, and for removing Pb from Zn–Ag alloy.
- Fractional distillation process: This process is used to purify metals which are volatile and the impurities in them are nonvolatile or vice versa. Zn, Cd and Hg are purified by this process.
- Zone refining method (Fractional crystallisation method): This process is used when metals are required to have very high purity for specific applications. For example, pure Si and Ge are used in semiconductors.
Chemical methods:
- Oxidative refining:
The molten impure metal is subjected to oxidation by various ways. This method is used for refining metals such as Pb, Ag, Cu and Fe. - Poling process:
This method is used for the purification of copper and tin which contain their own oxides as impurities.
Green wood→ Hydrocarbons →CH4
4CuO + CH4 → 4Cu (pure metal) + CO2 + 2H2O - Electrolytic refining:
Metals such as Cu, Ni and Al are refined electrolytically. - Vapour phase refining:
- Extraction of nickel (Mond’s process):
H2O(g) + C → CO(g) + H2 - van Arkel–de Boer process:
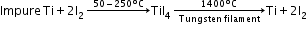
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry


