Chemical Bonding and Molecular Structure
Chemical Bonding and Molecular Structure PDF Notes, Important Questions and Synopsis
SYNOPSIS
- The attractive force which holds various constituents (atoms and ions) together in different chemical species is known as chemical bond.
- Octet Rule-According to the electronic theory of valence, every atom tries to attain the octet configuration (presence of eight electrons) in its valence shell by losing, gaining or sharing of electrons. This is known as the octet rule
- Lewis Symbols- To understand the concept of valence electrons, Lewis introduced the concept representing valence electrons with dots, which are called Lewis symbols.
- Electrovalent Bond- The bond formed as a result of the electrostatic attraction between positive and negative ions is termed the electrovalent bond. The electrovalence is thus equal to the number of unit charge(s) on the ion.
- Covalent Bond- When two same or different atoms share their valence electron to attain the noble gas configuration, it is known as a covalent bond.
- Formal Charge- The formal charge of an atom in a polyatomic molecule or ion may be the difference between the number of valence electrons of that atom in an isolated or free state and the number of electrons assigned to that atom in the Lewis structure.
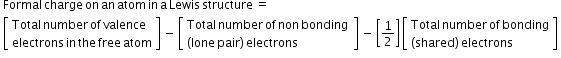
- Hybridisation: Mixing of two atomic orbitals with the same energy level to give a degenerated new type of orbitals Bond Length- Bond length is the equilibrium distance between the nuclei of two bonded atoms in a molecule.
- Bond Angle- Bond angle is the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion.
- Bond Enthalpy- Bond enthalpy is the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
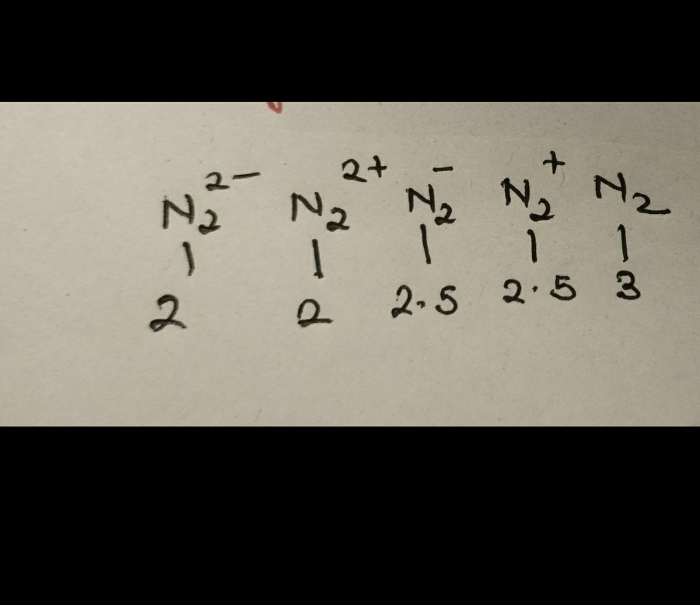
- Bond Order- According to Lewis, bond order is given by the number of bonds between two atoms in a molecule.
- Valence Bond Theory- Valence bond theory says that electrons in a covalent bond reside in a region which is at the overlap of individual atomic orbitals.
- Sigma (σ) bond- The covalent bond formed because of overlapping of atomic orbitals along the internuclear axis is called the σ-bond.
- Pi (π) bond- The covalent bond formed by sidewise overlapping of atomic orbitals is called π-bond.
- VSEPR Theory- The Lewis concept is unable to explain the shapes of molecules. Sidgwick and Powell provided a useful idea for predicting shapes and geometries of molecules. The theory was based on the repulsions between electron pairs, known as valence shell electron pair repulsion (VSEPR) theory.
|
e− Pairs
|
Notation |
Name of VSEPR shape |
Examples |
Reason for the acquired shape |
|
2 |
AX2 |
Linear |
HgCl2, ZnI2, CS2, CO2 |
|
|
3 |
AX3 |
Trigonal planar |
BF3, GaI3 |
|
|
AX2E |
Non-linear |
SO2, SnCl2 |
Theoretically, the shape should have been triangular planar, but actually it is bent or v-shaped. The reason being the lone pair–bond pair repulsion is much more as compared to the bond pair–bond pair repulsion. So, the angle is reduced to 119.5° from 120°. |
|
|
4 |
AX4 |
Tetrahedral |
CCl4, CH4, |
|
|
AX3E |
Trigonal pyramidal |
NH3, |
Had there been a bp instead of a lp, the shape would have been tetrahedral; but one lone pair is present, and because of the repulsion between lp–bp (which is more than bp–bp repulsion), the angle between the bond pairs is reduced to 107° from 109.5°. |
|
|
AX2E2 |
Non-linear |
H2O, SeCl2 |
The shape should have been tetrahedral if there were all bp, but two lp are present, so the shape is distorted tetrahedral or angular. This is because lp–lp repulsion is more than lp–bp repulsion which is more than bp–bp repulsion. Thus, the angle is reduced to 104.5° from 109.5°. |
|
|
5 |
AX5 |
Trigonal bipyramidal |
PCl5, PF5 |
|
|
AX4E |
Distorted tetrahedral |
TeCl4, SF4 |
The lp is in an equatorial position, and there are two lp–bp repulsions. Hence, it is more stable. |
|
|
AX3E2 |
T-shaped |
ClF3, BrF3 |
The lp is at an equatorial position, so there are less lp–bp repulsions as compared to others in which the lp is at the axial position. So, a T-shaped structure is the most stable. |
|
|
AX2E3 |
Linear |
, |
|
|
|
6 |
AX6 |
Octahedral |
SF6, |
|
|
AX5E |
Square pyramidal |
IF5, BrF5 |
|
|
|
AX4E2 |
Square planar |
|
|
- Hybridisation: Mixing of two atomic orbitals with the same energy level to give a degenerated new type of orbitals.
Hybridisation
Geometry
sp
Linear
sp2
Trigonal Planar
sp3
Tetrahedral
sp3d
Trigonal bipyramidal
sp3d2
Octahedral
sp3d3
Pentagonal bipyramidal
- Molecular Orbital Theory- According to Molecular Orbital Theory, individual atoms combines to form molecule. Thus the electrons of an atom are present in various atomic orbitals and are associated with several nuclei.
-
Energy Level Diagram for Molecular Orbitals
Contours and energies of bonding and antibonding molecular orbitals formed through combinations of 1s atomic orbitals, 2pz atomic orbitals and 2px atomic orbitals are shown below.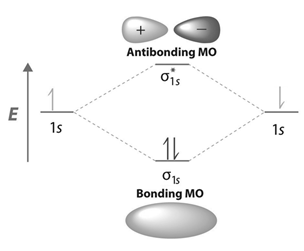
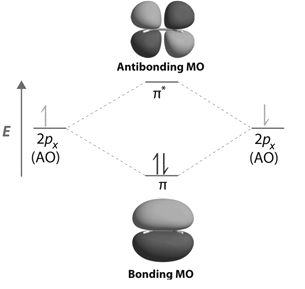
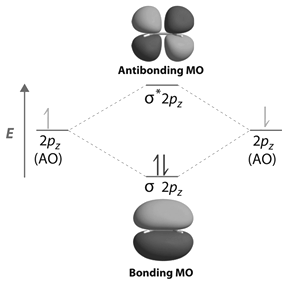
-
Hydrogen Bond- The hydrogen bond is a special type of attraction between a hydrogen atom bonded to a strongly electronegative atom (such as nitrogen, oxygen or fluorine) and the unshared pair of electrons on another electronegative atom.
-
Vander wall Interactions- Vander wall interactions are weakest of all bondings. These are driven by induced electrical interaction between two or more atoms or molecules.
-
Lattice Enthalpy- The lattice enthalpy of an ionic solid is the energy required to completely separate one mole of a solid ionic compound into gaseous constituent ions.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry

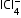 ,
,