Polymers
Polymers PDF Notes, Important Questions and Synopsis
SYNOPSIS
The word ‘polymer’ is coined from two Greek words. They are very large molecules having high molecular mass (103-107u). They are also called as macromolecules which are formed by joining of repeating structural units on a large scale.
- Monomer: The repeating structural units which are derived from some simple and reactive molecules are known as monomers. They are linked to each other by covalent bonds.
- Polymerisation: The process of formation of polymers from respective monomers is called polymerisation.
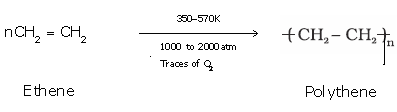
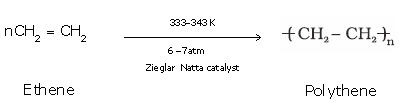
Example:Conversion of ethene to polyethene
Classification of Polymers
Based on some special considerations following are some of the common classifications of polymers:
- Based Classification Based on Source
In this type of classification, there are three sub categories:
- Natural Polymers
Proteins, cellulose, starch, some resins and rubber are examples of polymers found in plants. - Semi-synthetic Polymers
Cellulose derivatives like cellulose acetate (rayon) and cellulose nitrate etc. are examples of this category. - Synthetic Polymers
Plastic (polythene), nylon (6, 6) and synthetic rubbers (Buna –S) are examples of man-made polymers.
on some special considerations following are some of the common classifications of polymers:
- Classification Based on Structure of Polymers
- Linear Polymers: These polymers are made up of long and straight chains.
Examples: High density polythene, polyvinyl chloride - Branched Chain Polymers: They contain linear chains having some branches.
Example: Low density polythene - Cross linked or Network Polymers: It is formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains. Example: Bakelite, melamine etc.
Polymers can also be classified on the basis of mode of polymerisation into two sub groups.
- Addition Polymers
The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers.
Example: Formation of polythene from ethene.
The addition polymers formed by the polymerisation of two different monomers are known as copolymers.
Example: Buna-S, Buna-N, etc. - Condensation Polymers
These polymers are formed by repeated condensation reaction between two different bi-functional or tri-functional monomeric units.
- Classification Based on Molecular Forces
Polymers can also be classified on the basis of mode of polymerisation into two sub groups.
- Addition Polymers
The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers.
Example: Formation of polythene from ethene.
The addition polymers formed by the polymerisation of two different monomers are known as copolymers.
Example: Buna-S, Buna-N, etc. - Condensation Polymers
- These polymers are formed by repeated condensation reaction between two different bi-functional or tri-functional monomeric units.
- Classification Based on Molecular Forces
- Elastomers
- These are rubber solids with elastic properties.
- In these elastomeric polymers, the polymer chains are held together by weakest intermolecular forces which allow the polymer to be stretched.
Example: Buna-S, Buna-N, neoprene etc
- Fibres
- They are thread forming solids with high tensile strength and high modulus.
Example: Polyamides (nylon 6, 6), polyesters (terylene), etc.
- They are thread forming solids with high tensile strength and high modulus.
- Thermoplastic polymers
-
These are the linear or slightly branched long chain molecules which soften on heating and harden on cooling.
Example: polythene, polystyrene, polyvinyls, etc.
-
-
Thermosetting polymers
They are cross linked or heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible and cannot be reused.
Example: Bakelite, urea-formaldehyde resins etc.
Types of Polymerisation Reactions
Two broad types of polymerisation reactions:
- Addition or chain growth polymerisation
- Condensation or step growth polymerisation
Some important addition polymers
|
No. |
Name of polymer |
Polymerisation Reaction and Uses |
|
1 |
Low density polythene (LDP) |
Uses: It is used in the insulation of electricity-carrying wires and the manufacture of squeeze bottles, toys and flexible pipes. |
|
2 |
High density polythene (HDP) |
Uses: It is used for manufacturing buckets, dustbins, bottles, pipes etc. |
|
3 |
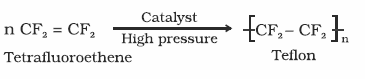
Polytetraflu-oroethene (Teflon) |
Uses: It is used in making oil seals and gaskets and also used for non-stick surface-coated utensils. |
|
4 |
Polyacrylon-itrile |
Uses: It is used as a substitute for wool in making commercial fibres as orlon or acrilan. |
- Condensation or step growth polymerisation
- In this type of polymerisation, there is a repetitive condensation reaction between two bi-functional monomers with the loss of some simple molecules like water leading to the formation of high molecular mass condensation polymers.
- Interaction of ethylene glycol and terephthalic acid leading to the formation of terylene is an
example of this type of condensation.
Some Important Condensation Polymerisation
- Polyamides
They are prepared by condensation polymerisation of diamines with dicarboxylic acids and also of amino acids and their lactams.Preparation of Nylon
Preparation of Nylons- Nylon 6,6
- It is prepared by the condensation polymerisation of hexamethylenediamine with adipic acid under high pressure and at high temperature.
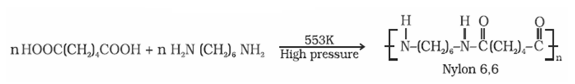
- It is used in making sheets, bristles for brushes and in textile industry.
- It is prepared by the condensation polymerisation of hexamethylenediamine with adipic acid under high pressure and at high temperature.
- Nylon 6
- It is obtained by heating caprolactum with water at a high temperature.
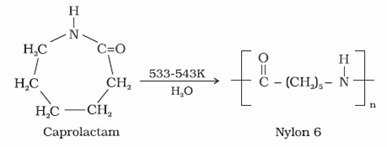
- It is used for the manufacture of tyre cords, fabrics and ropes.
- Nylon 6,6
- Polyesters
- They are obtained by the condensation products of dicarboxylic acids and diols.
- It is manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to 460K in the presence of zinc acetate-antimony trioxide catalyst.
- Phenol-formaldehyde polymer(Bakelite and related polymers)
- It is obtained by the condensation reaction of phenol with formaldehyde in the presence of either an acid or a base catalyst.
- The reaction proceeds with the initial formation of o-and/or p-hydroxy methyl phenol derivatives which further react with phenol to form compounds having rings joined to each other through –CH2 groups.
- The initial product could be a linear product – Novolac used in paints.
-
Melamine-formaldehyde polymer
It is formed by the condensation polymerisation of melamine and formaldehyde.
It is used in the manufacture of unbreakable crockery.
- Copolymerisation
- The copolymer can be prepared by both chain growth polymerisation and step growth polymerisation.
- It contains multiple units of each monomer used in the same polymeric chain.
- A mixture of 1, 3-butadiene and styrene can form a polymer.
- Rubber
- Natural Rubber
- Rubber is a natural polymer with elastic properties.
- It is prepared from rubber latex which is a colloidal dispersion of rubber in water.
- It can be considered as a linear polymer of isoprene (2-methyl-1, 3-butadiene) and is also called as cis-1, 4-polyisoprene.
Vulcanization of Rubber- This process is used to improve the physical properties of rubber.
- In this process, mixture of raw rubber is heated with sulphur and appropriate additive at a temperature range between 373 K to 415 K.
- Synthetic Rubbers
Polymers of Commercial Importance
Name of Polymer
Monomer
Structure
Uses
Polypropene
Propene
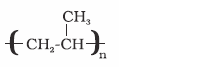
Manufacture of
ropes, toys, pipes,
fibres etc.
Polystyrene
Styrene
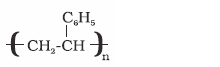
As insulator, wrapping
material, manufacture
of toys, radio and
television cabinets
Polyvinyl chloride
(PVC)
Vinyl chloride
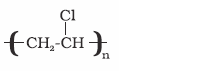
Manufacture of rain
coats, hand bags, vinyl flooring, water pipes
Glyptal
(a) Ethylene glycol
(b) Phthalic acid
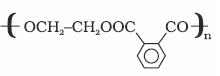
Manufacture of
paints and lacquers
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry