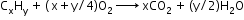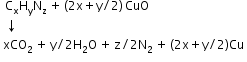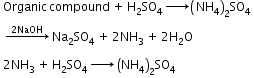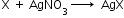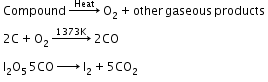Purification and Characterisation of Organic Compounds
Purification and Characterization of Organic Compounds PDF Notes, Important Questions and Synopsis
SYNOPSIS
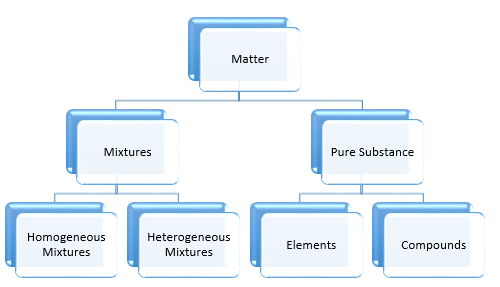
- Classification of organic compounds:
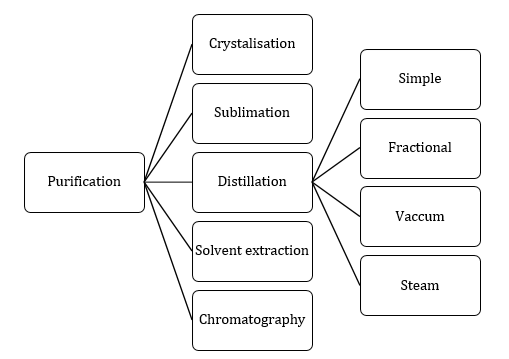
- Purification of organic compounds:
-
Filtration: It is the technique used to separate an insoluble solid component of the mixture from a soluble component in a given solvent.
-
Recrystallisation: This method is based on the differences in the solubility of the organic compound and its impurities in a suitable solvent.
-
Simple distillation: This method is used for the purification of liquids which boils without decomposition and contain non-volatile impurities.
-
Fractional distillation: Method is used to separate a mixture of two or more miscible liquids which have boiling points close to each other. Distillation is carried out by using fractionating columns.
Distillation under reduced pressure or vacuum distillation: Certain liquids have a tendency to decompose at a temperature below their boiling points. Under reduced pressure, the liquid will boil at a low temperature and the temperature of decomposition will not be reached. -
Steam distillation: This method is used for the separation and purification of a liquid which is appreciably volatile in steam from non-volatile components of a mixture.
Differential extraction: This method is used to separate a given organic compound present in aqueous solutions by shaking with a suitable organic solvent in which the compound is more soluble than water. The basic requirement of the organic solvent is that it should be immiscible with water so that the organic and water layers can be easily separated. -
Chromatography: The technique of chromatography is based on the difference in the rates at which the components of a mixture move through a porous medium (called stationary phase) under the influence of some solvent or gas (called moving or mobile phase).
Based on the principle involved, chromatography is classified as(a) Adsorption chromatography: It is based on the fact that different compounds are adsorbed on an adsorbent to different degrees. When a mobile phase is allowed to move over a stationary phase (adsorbent), the components of the mixture move by varying distances over the stationary phase because of the different adsorption tendencies.
(i) Column chromatography: In this method, a suitable adsorbent such as alumina (Al2O3), silica (SiO2) or Fuller’s earth is packed as a column in a burette-like long tube and acts as a stationary phase. The mixture to be separated is dissolved in a suitable solvent, and the solution is poured on the top of the column of the adsorbent. The component which is adsorbed strongly gets adsorbed at the top.
(ii) Thin layer chromatography: This method involves separation of a mixture of substances over a thin layer of an adsorbent coated on a glass plate. After drying the glass plate is placed in a closed jar containing eluant. As the eluant rises up the plate, the components of the mixture move up along with the eluant to different distances depending on their degree of adsorption and separation takes place.
(b) Partition chromatography: This method is based on differences in tendencies of substances to distribute or partition between the stationary phase and mobile phase. Paper chromatography is a type of partition chromatography in which the mixture to be separated is applied in the form of a drop on the paper. This paper is then suspended in a suitable solvent or mixture of solvents. The solvent rises up by capillary action. Paper selectively retains different components according to their differing partition in two phases.
-
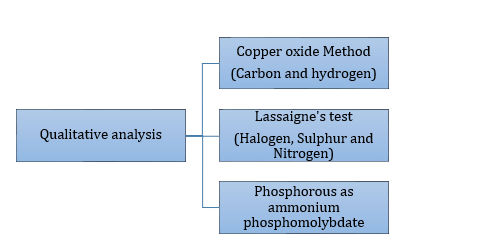
Qualitative Analysis of Organic Compounds: Analysis involving detection of all elements present in an organic compound.
-
Qualitative analysis (Detection of elements):
- Detection of Carbon and hydrogen by copper oxide test:
- Detection of Nitrogen, halogen and sulphur by Lassaigne’s test:
- Detection of carbon and hydrogen: Carbon and hydrogen are detected by heating the compound with copper (II) oxide. Carbon present in the compound is oxidised to carbon dioxide and hydrogen to water. Carbon dioxide is tested with lime water. Carbon dioxide makes lime water milky and develops turbidity. Water is tested with anhydrous copper sulphate, which becomes blue on absorbing moisture.
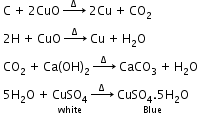
- Detection of nitrogen: The sodium fusion extract (prepared by boiling fused mass of compound and sodium metal in distill water) is boiled with FeSO4 (iron (II) sulphate) and then acidified with concentrated sulphuric acid. The formation of prussian-blue colour confirms the presence of nitrogen.
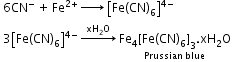
- Detection of sulphur: Two tests can be performed for detection of sulphur.
(a) On adding lead acetate to acidified sodium fusion extract, the formation of black precipitate confirms the presence of sulphur.
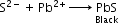
(b) On adding sodium nitroprusside to acidified sodium fusion extract, appearance of violet colour indicates the presence of sulphur.
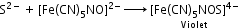
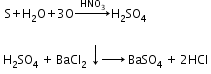
- Detection of halogens: On adding a silver nitrate solution to sodium fusion extract acidified with nitric acid white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate, sparingly soluble in ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble in ammonium hydroxide shows the presence of iodine.
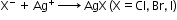
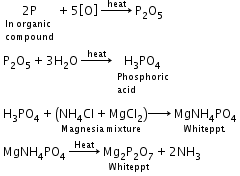
- Detection of phosphorous: Compound containing phosphorous is heated with an oxidizing agent (sodium peroxide) which oxidises phosphorus to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
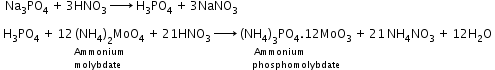
- Detection of carbon and hydrogen: Carbon and hydrogen are detected by heating the compound with copper (II) oxide. Carbon present in the compound is oxidised to carbon dioxide and hydrogen to water. Carbon dioxide is tested with lime water. Carbon dioxide makes lime water milky and develops turbidity. Water is tested with anhydrous copper sulphate, which becomes blue on absorbing moisture.
-
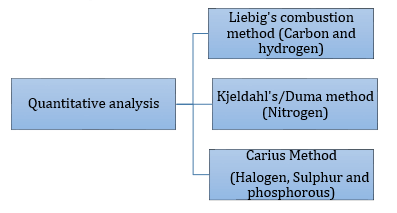
Quantitative Analysis of Organic Compounds: It is the analysis that involves the determination of percentages of various elements present in a given compound.
-
Quantitative analysis:
-
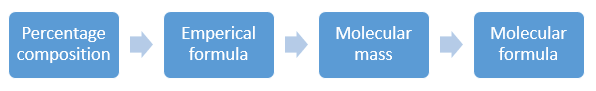
Relation between Empirical and molecular formula:

-
Determination of Empirical and molecular formula:
Principle involved in estimation of various elements
|
||
|
||
|
Duma’s method: A known mass of an organic compound is heated with dry cupric oxide in an atmosphere of carbon dioxide. If any oxide of nitrogen is produced during this process, then it is reduced to nitrogen by passing over heated copper gauze. The gaseous mixture is collected over an aqueous solution of KOH, when all the gases except nitrogen are absorbed. The volume of nitrogen produced is measured at room temperature and atmospheric pressure. |
Kjeldahl’s method: A known mass of the organic compound is heated with concentrated H2SO4 so that nitrogen is quantitatively converted into ammonium sulphate. The resulting solution is then heated with excess of sodium hydroxide. The ammonia gas evolved is passed into a known but excess volume of standard acid (HCl or H2SO4). The acid left unused is estimated by titrating the solution with standard alkali. From the amount of acid left unused, the amount of acid used for neutralization of ammonia can be calculated. |
|
|
||
|
||
|
Phosphorus:A known mass of an organic compound is heated with fuming nitric acid. Due to this phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, (NH4)3PO4.12MoO3, by adding ammonia and ammonium molybdate. This is filtered, washed, dried and weighed.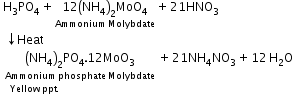 |
|
|
Oxygen: A definite amount of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red hot coke when oxygen gets converted to carbon monoxide. The mixture is then passed through warm iodine pentoxide (I2O5) when carbon monoxide is oxidised to carbon dioxide producing Oxygen is generally estimated by subtracting the sum of the percentage of all other elements in the compound from 100. |
||
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry