CBSE Class 11-science Answered
Write electronic configuration of following elements in
form of orbital notation and orbital diagram C
Asked by salvadormiranda2509 | 13 Feb, 2021, 12:53: PM
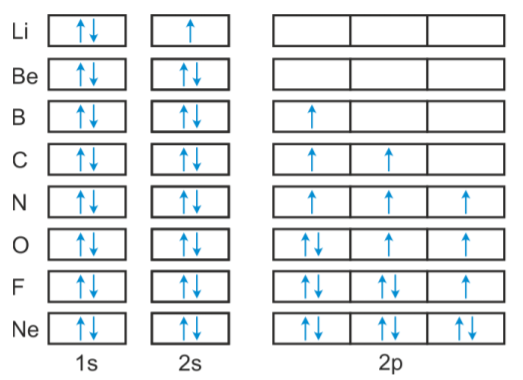
- The electronic configuration of Li is 1s2 2s1 .
- The 2s orbital can accommodate one more electron.
- The configuration of beryllium (Be) atom is therefore 1s2 2s2 .
- In the next six elements—boron (B, 1s2 2s2 2p1 ), carbon (C, 1s2 2s2 2p2 ), nitrogen (N, 1s2 2s2 2p3 ), oxygen (O, 1s2 2s2 2p4 ), fluorine (F, 1s2 2s2 2p5 ) and neon (Ne, 1s2 2s2 2p6 ), the 2p orbitals get progressively filled.
- This process is completed with the neon atom. The orbital picture of these elements can be represented as follows:
Answered by Ramandeep | 15 Feb, 2021, 14:32: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by Neetukhaiwal | 24 Jul, 2021, 11:18: AM
CBSE 11-science - Chemistry
Asked by salvadormiranda2509 | 13 Feb, 2021, 12:53: PM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 01 May, 2020, 16:37: PM
CBSE 11-science - Chemistry
Asked by prashantyadav592 | 29 Jan, 2020, 09:39: AM
CBSE 11-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:47: PM
CBSE 11-science - Chemistry
Asked by sairamsribaba80 | 28 Aug, 2019, 18:54: PM
CBSE 11-science - Chemistry
Asked by timeyashkadam | 19 Apr, 2019, 10:22: AM
CBSE 11-science - Chemistry
Asked by himankarbhati3 | 21 Sep, 2018, 08:29: AM
CBSE 11-science - Chemistry
Asked by ramandeep.kaur | 06 Sep, 2018, 13:21: PM