CBSE Class 11-science - Electronic Configuration of Atoms Videos
Structure of Atom
This video explains shapes of atomic orbitals, Aufbau principle and Pauli's exclusion principle.
More videos from this chapter
View All- what is atomic orbital?
- Arrange the electrons represented by the following set of quantum number in decreasing order of energy. (i) n = 4, l =0, m=0 S= + (ii) n = 2, l =1, m=1 S= - (iii) n = 3, l =2, m=0 S= + (iv) n = 3, l =0, m=0 S= -
- Write electronic configuration of following elements in form of orbital notation and orbital diagram C
-
pls solve
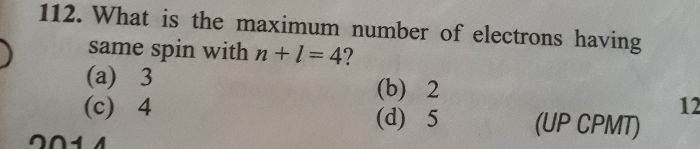
- Why first ionisation enthalpy of Mg is higher than Al
- Why zeff less than screening effect for copper and zinc
- show the electron distribution in fluorine atom.how many unpaired electrons are there in fluorine
- HOW TO FIND VALENCY USING spdf METHOD ?IS IT POSSIBLE?
- Difference between orbit and orbitals
- Explain in detail electronic configuration of atoms.






