CBSE Class 11-science - Electronic Configuration of Atoms Videos
Structure of Atom
This video explains Hund's rule of maximum multiplicity, electronic configuration, exceptional electronic configuration and stability of half and completely filled subshells.
More videos from this chapter
View All- what is atomic orbital?
- Arrange the electrons represented by the following set of quantum number in decreasing order of energy. (i) n = 4, l =0, m=0 S= + (ii) n = 2, l =1, m=1 S= - (iii) n = 3, l =2, m=0 S= + (iv) n = 3, l =0, m=0 S= -
- Write electronic configuration of following elements in form of orbital notation and orbital diagram C
-
pls solve
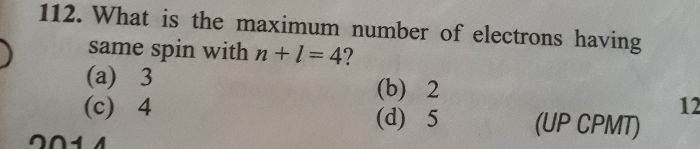
- Why first ionisation enthalpy of Mg is higher than Al
- Why zeff less than screening effect for copper and zinc
- show the electron distribution in fluorine atom.how many unpaired electrons are there in fluorine
- HOW TO FIND VALENCY USING spdf METHOD ?IS IT POSSIBLE?
- Difference between orbit and orbitals
- Explain in detail electronic configuration of atoms.






