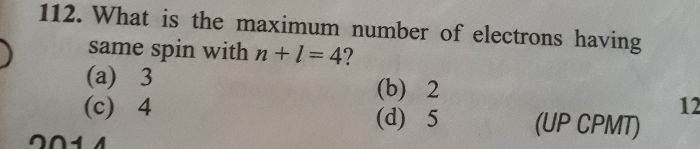CBSE Class 11-science Answered
electronic configuration of elements
Asked by | 04 Mar, 2008, 05:57: PM
As per aufbaus principle, paulis exclusion principle and hunds rule are applied to write electronic configuration of elements. Lower energy levels are filled first and than the higher levels as per aufbaus principle.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s and so on the orbitals will be filled. s will have maximum 2 , p will have 6 , d will have 10 and f will have 14 electrons.
Answered by | 20 Dec, 2017, 04:55: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by Neetukhaiwal | 24 Jul, 2021, 11:18: AM
CBSE 11-science - Chemistry
Asked by salvadormiranda2509 | 13 Feb, 2021, 12:53: PM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 01 May, 2020, 04:37: PM
CBSE 11-science - Chemistry
Asked by prashantyadav592 | 29 Jan, 2020, 09:39: AM
CBSE 11-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:47: PM
CBSE 11-science - Chemistry
Asked by sairamsribaba80 | 28 Aug, 2019, 06:54: PM
CBSE 11-science - Chemistry
Asked by timeyashkadam | 19 Apr, 2019, 10:22: AM
CBSE 11-science - Chemistry
Asked by himankarbhati3 | 21 Sep, 2018, 08:29: AM
CBSE 11-science - Chemistry
Asked by ramandeep.kaur | 06 Sep, 2018, 01:21: PM