CBSE Class 12-science Chemistry Three Dimensional Packing
Let us help you in your efforts to score more marks in the board exams with our CBSE Class 12 Science Chemistry The Solid State – Three-Dimensional Packing. Understand the concept of close packing in 3D with our video lessons. To revise the chapter concepts before the exams, you can read the explanations in our topic notes. Respected Chemistry experts have developed these notes to support you in your Chemistry exam preparation.
On the TopperLearning study portal, you will also get online textbook solutions such as NCERT solutions for revision. To practise questions related to Three-Dimensional Packing, check our CBSE Class 12 Science Chemistry sample papers, previous years’ papers and practice tests.
- what is solid
- The unit cell of Na is bcc and its density is 0.97. What is the radius of a sodium atom if the molar mass of Na is 23?
-
explain please

-
pls solve sir
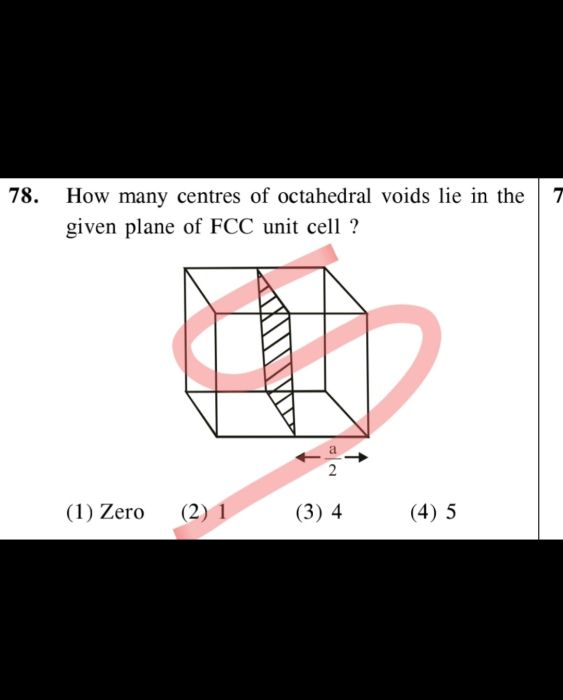
- How many types of 3dimensions are there in a lattice please define.
- sir in types of unit cell we studied it can be of 4 types i.e primitive, face centred, body centred and end centred, but while studing 3D close packing it was told that it can be of 3 types i,e. simple(primitive) cubic unit cell, hcp and ccp. but i am not able to understand that hcp is a type of unit cell or it is one of the 7 crystal system or somthing else?and if it is type of unit cell then why it is not taught under the topic types of unit cell. and if it is a crystal system then what about the rest 6 crystal system, then why rest 6 crystal system does not comes under 3 dimensional close packing?
- In a cubic lattice A atoms having FCC arrangement B atoms are at body centers and C atoms are at alternate edge centers . The formula of the unit cell will be ;
- The number of c4 and c3- axes in a cubic crystal lattice are x and y respectively the value of x+y= a] 2 b] 7
- Hexagonal close packing and cubic close packing
- Analysis shows that nickel oxide has the formula ni 0.98 o1.00. What fractions of nickel exist as ni 2+ and ni 3+ ions?




