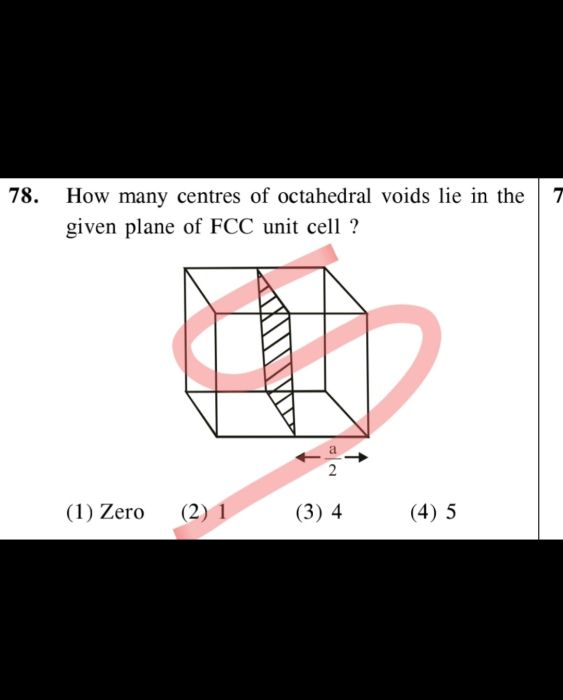
CBSE Class 12-science Answered
The unit cell of Na is bcc and its density is 0.97. What is the radius of a sodium atom if the molar mass of Na is 23?
Asked by arushidabhade | 17 Mar, 2021, 13:24: PM
Density in unit cell can be determined by-
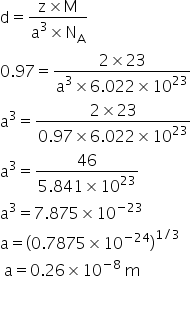
Answered by Ravi | 20 Mar, 2021, 01:49: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by harshul2019 | 25 May, 2022, 20:52: PM
CBSE 12-science - Chemistry
Asked by arushidabhade | 17 Mar, 2021, 13:24: PM
CBSE 12-science - Chemistry
Asked by kasthurikalvi | 16 Sep, 2020, 15:46: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 01 Jul, 2020, 22:08: PM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 18 May, 2020, 15:00: PM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 10 Sep, 2019, 19:10: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 05 Aug, 2019, 00:08: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 04 Aug, 2019, 21:06: PM
CBSE 12-science - Chemistry
Asked by ranasingh04082002 | 31 Jul, 2019, 14:04: PM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 18:51: PM