CBSE Class 12-science Answered
Analysis shows that nickel oxide has the formula ni 0.98 o1.00. What fractions of nickel exist as ni 2+ and ni 3+ ions?
Asked by Saransekar407 | 11 Mar, 2019, 18:51: PM
Given formula is Ni0.98O1.0
the ratio of Ni : O = 98 : 100 which means for 100 atoms of oxygen there are 98 atoms of nickels are present.
Let us consider the number of atoms of Ni2+ = x hence the number of atoms of Ni3+ = 98 - x
Charge on Ni = charge on O
3(98-x) + 2x = 2(100)
294 - 3x + 2x = 200
-x = -94
x = 94
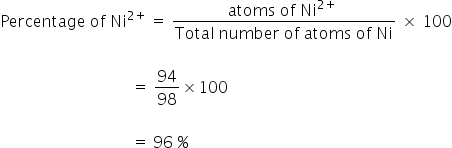
Percentage of Ni+3 = 100 - Ni2+
= 100 - 96
= 4 %
Answered by Ramandeep | 11 Mar, 2019, 19:13: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by harshul2019 | 25 May, 2022, 20:52: PM
CBSE 12-science - Chemistry
Asked by arushidabhade | 17 Mar, 2021, 13:24: PM
CBSE 12-science - Chemistry
Asked by kasthurikalvi | 16 Sep, 2020, 15:46: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 01 Jul, 2020, 22:08: PM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 18 May, 2020, 15:00: PM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 10 Sep, 2019, 19:10: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 05 Aug, 2019, 00:08: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 04 Aug, 2019, 21:06: PM
CBSE 12-science - Chemistry
Asked by ranasingh04082002 | 31 Jul, 2019, 14:04: PM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 18:51: PM