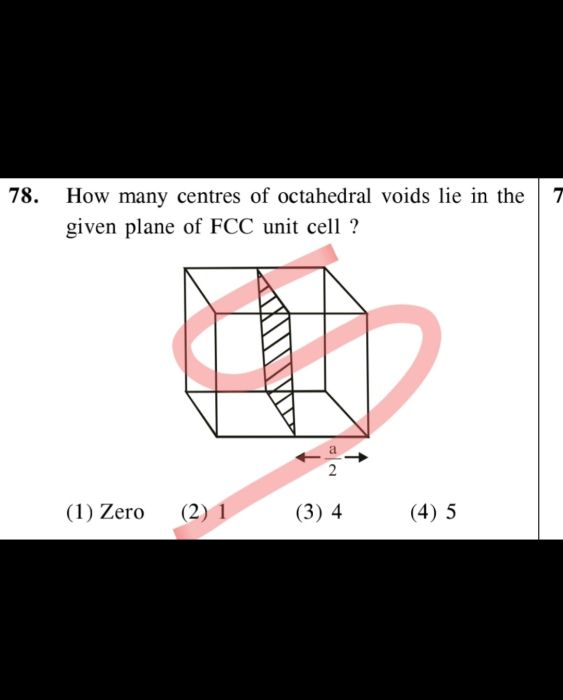
CBSE Class 12-science Answered
The number of c4 and c3- axes in a cubic crystal lattice are x and y respectively the value of x+y=
a] 2
b] 7
Asked by prakriti12oct | 04 Aug, 2019, 21:06: PM
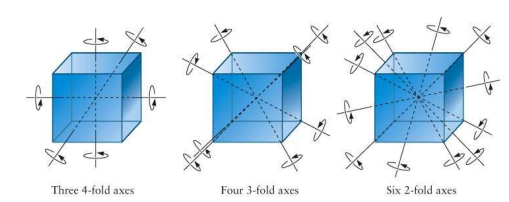
These are C4, C3 and C2 axes shown by a cubical lattice.
Answered by Ramandeep | 05 Aug, 2019, 15:03: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by harshul2019 | 25 May, 2022, 20:52: PM
CBSE 12-science - Chemistry
Asked by arushidabhade | 17 Mar, 2021, 13:24: PM
CBSE 12-science - Chemistry
Asked by kasthurikalvi | 16 Sep, 2020, 15:46: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 01 Jul, 2020, 22:08: PM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 18 May, 2020, 15:00: PM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 10 Sep, 2019, 19:10: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 05 Aug, 2019, 00:08: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 04 Aug, 2019, 21:06: PM
CBSE 12-science - Chemistry
Asked by ranasingh04082002 | 31 Jul, 2019, 14:04: PM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 18:51: PM