Periodic Table
Periodic Table Synopsis
Synopsis
Periodic Classification of Elements
Early Attempts of Classification of Elements
Dobereiner’s Triads
Law of Triads: When elements are arranged in the order of their increasing atomic masses, the atomic mass of the middle element was approximately the mean of the atomic masses of the other two elements.
For example:
Consider the triad of lithium, sodium and potassium. The atomic mass of sodium is the mean of the atomic masses of lithium and potassium.
Newlands’ Law of Octaves
- Law of Octaves:
When elements are arranged in the increasing order of their atomic masses, the properties of every eighth element is similar to the first.
Limitations - Newland could arrange elements only up to calcium, out of the total 56 elements known.
- After calcium, every eighth element did not possess properties similar to that of the first.
- Only 56 elements were known at the time of Newland, but later several new elements were discovered.
- In order to fit the existing element arrangement, Newland placed two elements in the same position which differed in their properties.
- For example: Iron, an element which resembles cobalt and nickel in its properties is placed far away from these elements.
- The periodic table did not include inert gases because they were not discovered then.
Mendeleev’s Periodic Table
- Mendeleev’s Periodic Law: The physical and chemical properties of elements are a periodic function of their atomic masses.
Features of Mendeleev’s Periodic Table
- There are seven horizontal rows in the periodic table, numbered from 1 to 7. These seven rows are called periods.
- There are eight vertical columns numbered from I to VIII. These eight columns are called groups. Groups I to VII are further divided into sub groups A and B.
- The properties of elements in a particular period show regular gradation from left to right.
Merits of Mendeleev’s Periodic Table
- Mendeleev kept some blank spaces in the periodic table for the elements which were yet to be discovered.
- He also predicted properties of some elements even before their discovery which were later found to be correct.
- Mendeleev’s periodic table could accommodate noble gases when they were discovered.
Demerits of Mendeleev’s Periodic Table
- Hydrogen resembles alkali metals as well as halogens. So, a correct position could not be assigned to hydrogen in the periodic table.
- The position of isotopes could not be explained. Isotopes are atoms of the same element having similar chemical properties but different atomic masses. If the elements are arranged according to atomic masses, the isotopes should be placed in different groups of the periodic table.
- At certain places, an element of higher atomic mass was placed before an element of lower atomic mass.
For example: Cobalt (Co = 58.93) was placed before nickel (Ni = 58.71). - Some elements placed in the same sub group had different properties.
For example: Manganese is placed with the halogens which are totally different in their properties
Modern Periodic Table
- In 1913, Henry Moseley proved that the atomic number is the fundamental property rather than its atomic mass.
- Modern Periodic Law: Properties of elements are a periodic function of their atomic numbers.
- The periodic table, based on the Modern Periodic Law is called the Modern Periodic Table.
Position of Elements in the Periodic Table
Periods
- The horizontal rows in the Modern Periodic Table are called periods.
- The Modern Periodic Table consists of seven periods which are numbered from 1 to 7.
- In each period, a new shell starts filling up. The period number is also the number of shell which starts filling up.
Groups
- The vertical columns are called groups and consist of eighteen groups numbered from 1 to 18.
- Elements having the same number of valence electrons are present in the same group.
- Elements present in the same group show the same chemical properties.
Properties in Modern Periodic Table
Periodicity
The properties that reappear at regular intervals, or in which there is gradual variation at regular intervals, are called periodic properties, and the phenomenon is known as the periodicity of elements.
Cause of Periodicity
The cause of periodicity is the recurrence of similar electronic configuration, i.e. the same number of electrons in the outermost orbit.
Shells (Orbits) and Valency
Orbits: Electrons revolve around the nucleus in certain definite circular paths called orbits or shells.
- Number of Shells
- Down a group, i.e. from top to bottom
The number of shells increases successively, i.e. one by one, such that the number of shells of an element equals the number of the period to which that element belongs.
Example: Halogens (Group 17 elements)
b.Across a period, i.e. from left to right
On moving from left to right in a given period, the number of shells remains the same.
For example, in the second period, the number of shells remains two, i.e. equal to the number of the period.
ii. Valency
- Valency denotes the combining capacity of the atom of an element. It is equal to the number of electrons an atom can donate or accept or share.
- On moving down a given group, the number of electrons in the outermost shell, i.e. valence electrons remains the same. So, the valency in a group also remains the same.
- In a given period, the number of electrons in the outermost shell increases from left to right. However, the valency increases only up to Group 14 where it becomes 4, and then, it decreases to 1 in Group17.
Periodic Properties
The properties which appear at regular intervals in the periodic table are called periodic properties, and the phenomenon is called periodicity in properties of elements.
Periodic Properties of Elements are
- Atomic size
- Metallic character
- Non-metallic character
- Ionisation energy
- Electron affinity
- Electronegativity
- Atomic Size (Atomic Radii)
It is the distance between the centre of the nucleus of an atom and its outermost shell.
Trends in Atomic Size
- Down a group
In a group, the size of an atom increases as one proceeds from top to bottom. This is due to the successive addition of shells as we move from one period to the next in a group.
In Group I, the size of hydrogen is the smallest and that of caesium is the largest.
H < Li < Na < K < Rb < Cs
37 pm 152 pm 186 pm 231 pm 244 pm 262 pm - Across a group
In a period, the size of an atom decreases from left to right. This is because the nuclear charge increases from left to right in the same period, thereby bringing the outermost shell closer to the nucleus. In the third period, sodium is the largest in size.
Na > Mg > Al > Si > P > S > Cl
186 pm 160 pm 143 pm 117 pm 110 pm 104 pm 99 pm
- Metallic Character
Elements which have a tendency to lose their valence electrons and form a positive ion are considered metals.
Na − e− → Na+
Trends in Metallic Character
- Down a group
On moving down a group, increased atomic size is greater as compared to the increased nuclear charge. Therefore, the metallic nature increases as one moves down a group, i.e. they can lose electrons easily. Example: In group 1, lithium is the least metallic element, while francium is the most metallic element. - Across a period
On moving across a period, the nuclear charge increases and the atomic size decreases, and hence, elements cannot lose electrons easily. Therefore, the metallic nature decreases across a period moving from left to right.
Example: In the 2nd period, lithium is the most metallic element.
- Non-Metallic Character
Elements which have a tendency to gain electrons in order to attain an octet in their outermost orbit are considered non-metals.
Example: Cl + e− → Cl−
(2, 8, 7) (2, 8, 8)
Trends in Non-metallic Character
- Down a group
The atomic size increases due to the addition of new shells over successive periods. Therefore, the non-metallic character decreases down the group.
Example: Group 14
C Non-metal
Si Metalloid
Ge Metalloid
Sn Metal
Pb Metal - Across a period
On moving across a period, the tendency to gain electrons increases due to an increase in nuclear pull and decrease in atomic size. So, the non-metallic character increases across a period, i.e. from left to right.
For example, in the 3rd period,
- Nature of Oxides
Trends in Nature of Oxides
- Across a period
Oxides of elements in a particular period show decreasing basic nature and finally become acidic. - Down a group
The basic nature of oxides of metals increases.
- Chemical Reactivity
In metals, greater the tendency to lose electrons, greater is the reactivity.
In non-metals, greater the tendency to gain electrons, greater is the reactivity.
Trends in Chemical Reactivity
- Across a period
On moving from left to right in a period, the chemical reactivity of elements first decreases and then increases.
Third period: - Down a group
The reactivity in metals increases on going down the group because the chemical reactivity in metals depends on the tendency to lose electrons.The chemical reactivity of non-metals decreases on going
the group as it depends on the tendency to gain electrons.
- Gradation in Physical Properties
The melting and boiling points of metals decrease on going down the group.
The melting and boiling points of non-metals increase on going down the group.
- Ionisation Energy
The energy required to remove an electron from a neutral isolated gaseous atom and to convert it into a positively charged gaseous ion is called ionisation energy (IE) or first ionisation energy (IE1).
Trends in Ionisation Energy
- Across a period
Ionisation energy tends to increase as one moves from left to right across a period, because the atomic size decreases due to an increase in the nuclear charge, and thus, more energy is required to remove the electrons.
- Down a group
Ionisation energy decreases with an increase in the atomic size, i.e. it decreases as one moves down a group.
- Electron Affinity or Electron Gain Enthalpy
The amount of energy released while converting a neutral gaseous isolated atom into a negatively charged gaseous ion by the addition of electrons is called electron affinity.
Trends in Electron Affinity
- Across a period
In a period, i.e. from left to right in a horizontal row of the periodic table, the atomic size decreases and the nuclear charge increases, so the electron affinity increases. - Down a group
Moving from the top to the bottom in a group, the atomic size increases more than the nuclear charge, thereby causing a net decrease in electron affinity.
- Electronegativity
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called its electronegativity.
Trends in Electronegativity
- Across a period
Electronegativity increases from left to right in a period, because the nuclear charge increases due to an increase in the atomic number.
Example: In the second period, electronegativity increases from lithium to fluorine. - Down a group
Electronegativity decreases down the group due to an increase in the atomic size overcoming the effect of an increase in the nuclear charge.
Atomic Number (Z) and Mass Number (A)
- The atomic number of an element is equal to the number of protons in the nucleus.
- The atomic number is a unique property of an element, because no two elements have the same atomic number.
- The mass number of an element is the sum of the number of protons and neutrons in the nucleus of the atom of the element.
Variation in Periodic Properties
4 Blocks in the Periodic Table
- The similarity among the elements in a vertical column (group) of the periodic table is due to the same number and same distribution of electrons in their outermost orbitals.
- The modern periodic table is divided into four main blocks depending on the type of orbital that are being filled with an exception of hydrogen and helium.
- This classification is based on the electronic configuration.
- The s-block contains 2 groups, the p-block contains 6 groups, the d-block contains 10 groups and the f-block contains 14 groups.
- The s-block is at the extreme left and the p-block is at the extreme right of the periodic table.
- The d-block is between the s-block and the p-block, and the f-block is separately placed below the main body of the table.
- Elements of the s-block and the p-block are collectively called representative elements or main block elements.
s-Block Elements
- Elements in which the last electron enters the s-orbital of the outermost energy level are called s-block elements.
- The s-block consists of two groups—Group 1 and Group 2.
- Elements of Group 1 are called alkali metals, and their general outer electronic configuration is ns1.
- Elements of Group 2 are called alkaline earth metals, and their general outer electronic configuration is ns2.
- They are reactive metals with low ionisation enthalpies.
- A quantitative measure of the tendency of an element to lose electrons is given by its ionisation enthalpy.
- The compounds of the s-block elements are predominantly ionic except lithium and beryllium.
- The elements of the s-block are strong reducing agents and good conductors of heat and electricity.
- These are
- Most reactive metals
- Most electropositive metals
- Strongly reducing in nature
- Strong tendency to lose electrons
Comparative study of Alkali metals and Alkaline Earth metals:
- Reaction with water:
All hydroxides of alkali metals are highly soluble in water and thermally stable, with the exception of LiOH.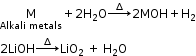
Hydroxides of alkali metals react with acids to give salts. The resultant salts are colourless, ionic solids which are soluble in water.
NaOH+HCl → Na Cl + H2O
2NaOH + H2SO4 → NaSO4 + 2 H2O
Alkaline earth metals, such as Ca, Sr and Ba, give their respective hydroxides when treated with cold water or reacted with their oxides.
M+2H2O →M (OH)2+ H2 (M=Ca,Sr,Ba)
MO + H2O →M(OH)2 (M= Ca,Sr,Ba) - Reaction with oxygen:
When alkali and alkaline earth metals are heated in the presence of oxygen, they give their respective oxides. Also, the thermal decomposition of their carbonates produces oxides.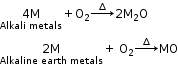
- Reaction with Hydrogen:
Alkali and alkaline earth metals reacts with hydrogen to form their stable hydrides.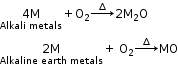
- Reaction with Chlorine:
All the alkali metals and alkaline earth metals react with chlorine to give their corresponding chlorides.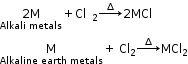
Download complete content for FREE 

