Industrial Chemistry
Industrial Chemistry Synopsis
Synopsis
Some important nonmetals:
Bonding in Carbon
- Carbon atom has four electrons in its outermost shell.
- It requires four electrons to achieve the stable, 8 electron, inert gas electron arrangement.
- Carbon atoms can achieve the inert gas electron arrangement only by sharing of the electrons. Hence, carbon always forms covalent bonds.
- The valency of carbon is four since one carbon requires 4 electrons to achieve the nearest inert gas configuration. Thus, we can say that carbon is tetravalent.
- The four valencies of carbon are usually represented by putting four short lines around the symbol of carbon, C.
Allotropes of Carbon
- The various physical forms in which an element can exist are called the allotropes of the element.
- Three allotropes of carbon are:
- Diamond
- In diamond, each carbon atom is bonded to four other carbon atoms, forming a three dimensional structure.
- The rigid structure of diamond makes it a very hard substance.
- It is a non-conductor of electricity since there are no free electrons in a diamond crystal.
- It can be synthesised by subjecting pure carbon to very high pressure and temperature.
- Graphite
- In graphite, each carbon atom is bonded to three other carbon atoms in the same plane, giving a hexagonal array.
- One of the bonds is a double bond and thus the valency of the carbon is satisfied.
- Graphite structure is formed by the hexagonal arrays being placed in layers, one above another.
- Graphite is smooth and slippery.
- It is a very good conductor of electricity due to the presence of free electrons.
- Fullerene:
- It is an allotrope of carbon containing clusters of 60 carbon atoms joined together to form spherical molecules.
- There are 60 carbon atoms in a molecule of buckminsterfullerene, so its formula is C60.
- The allotrope was named buckminsterfullerene after the American architect Buckminster Fuller.
Versatile Nature of Carbon
- The number of carbon compounds already known at present is more than 5 million.
- Every day more new compounds are isolated or prepared in the laboratories.
- The two characteristic properties of the carbon element which leads to the formation of a very large number of organic compounds are
- Catenation: The property of carbon element due to which its atoms can join one another to form long carbon chains is called catenation.
A) Straight chain of carbon atoms
B) Branched chain of carbon atoms
C) Closed chain or ring chain of carbon atoms
ii. Tetravalency: Carbon has a valency of four. So, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element. Compounds of carbon are formed with oxygen, nitrogen, hydrogen, sulphur, chlorine and many other elements, giving rise to compounds with specific properties which depend on the elements other than the carbon present in the molecule.
Compounds of Nitrogen:
Ammonia
Molecular formula: NH3
Relative molecular mass: 17 am
Lewis diagram or dot diagram
Occurrence
- Free State:
Ammonia is present in small amounts in air and in traces in natural water.
Combined state:
Ammonia occurs in the combined form in compounds such as ammonium chloride and ammonium sulphate.
i. Gaseous ammonia
ii. Liquid ammonia
iii. Liquor ammonia fortis
iv. Laboratory bench reagent
Preparation of Ammonia Gas
Laboratory Preparation
- From Ammonium Chloride
Reactants: Ammonium chloride (NH4Cl) and calcium hydroxide [Ca(OH)2] in the ratio of 2:3 by weight.
Reaction:
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
Diagram:
Drying of Ammonia Gas
- The gas is passed through a drying tower containing lumps of quicklime (CaO).
- Drying agents such as conc. sulphuric acid, phosphorous pentoxide and calcium chloride are not used because ammonia, being basic, reacts with them.
2NH3 + H2SO4 → (NH4)2SO4
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
CaCl2 + 4NH3 → CaCl2.4NH3
Collection
Ammonia gas is collected by the downward displacement of air because it is
- Lighter than air (VD of NH3 = 8.5 and that of air = 14.4)
- Highly soluble in water (so, it cannot be collected over water)
2. From Metal Nitrides
It can also be prepared by the action of warm water on nitrides of metals such as magnesium or aluminium.
Reaction:
AlN + 3H2O → Al(OH)3 + NH3
Or
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
Manufacture of Ammonia (Haber’s Process)
Reactants:
Nitrogen and hydrogen in the ratio of 1:3 by volume.
Reaction:
N2 + 3H2 ⇌ 2NH3 + Heat
Sources of Reactants
Nitrogen: It is obtained by fractional distillation of liquid air.
Hydrogen: It is obtained from water gas (Bosch process) or natural gas.
Favourable Conditions
Temperature: Optimum temperature is 450−500°C.
Pressure: Above 200 atm.
Catalyst: Finely divided iron
Promoter: Traces of molybdenum or Al2O3
Chemical Properties of Ammonia
Uses of Ammonia
- Liquid ammonia is used as a refrigerant in ice plants.
- Aqueous NH3 dissolves fat and grease, so it is used
a. To remove grease and sweat stains from clothes
b. For cleaning tiles, windows etc. - Used as a laboratory reagent in qualitative analysis because it produces characteristic coloured metallic hydroxide precipitates.
- Ammonia is used in the manufacture of
- Nitrogenous fertilisers such as ammonium sulphate and diammonium hydrogen phosphate
- Explosives such as ammonium nitrate
- Sodium carbonate by the Solvay process
- Nitric acid by the Ostwald process
Nitric Acid
Molecular formula: HNO3
Relative molecular mass: 63 amu
- Nitric acid was initially called ‘aqua fortis’.
- In 1658, Glauber obtained nitric acid by distilling nitre with sulphuric acid.
Occurrence
Free state: Nitric acid is found in rainwater in traces after lightning.
Combined state: As its salts in minerals
Examples: Chile saltpetre [NaNO3], Bengal saltpetre [KNO3] or nitre
Laboratory Preparation of Nitric Acid
Reactants:
Obtained by distilling conc. sulphuric acid with potassium KNO3 (nitre) or sodium NaNO3.
Reactions: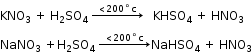
Collection
- Vapours of nitric acid are condensed into a light yellow liquid by chilling the receiver with running cold water.
- Pure nitric acid is colourless, while the nitric acid obtained in the laboratory is slightly yellowish-brown due to its decomposition, resulting in the formation of reddish-brown nitrogen dioxide.
- The yellow brown tinge in the acid can be removed by
- Bubbling of carbon dioxide, which oxidises nitrogen dioxide to nitric acid
- Dilution with water, which causes dissolution of water-soluble nitrogen dioxide
Precautions
- Glass apparatus is used because nitric acid vapours corrode rubber and cork.
- Conc. HCl is not used because it is volatile, and hence, nitric acid vapours will carry HCl vapours.
- The temperature of the reaction should not exceed 200°C because sodium sulphate formed at a higher temperature forms a hard crust which sticks to the walls of the retort and is difficult to remove.
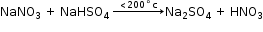
Manufacture of Nitric Acid
In 1914, a German chemist Ostwald developed the Ostwald process to manufacture nitric acid.
Step 1: Catalytic oxidation of ammonia
A mixture of dry air and dry ammonia in the ratio of 10:1 by volume is compressed and then passed into a platinum gauze which acts as a catalyst at about 800°C.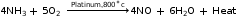
Step 2: Oxidation of nitric oxide
Nitric oxide combines with oxygen to form nitrogen dioxide at about 50°C.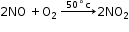
Step 3: Absorption of nitrogen dioxide in water
Nitrogen dioxide and oxygen present in the air react with water to form nitric acid.
4NO2 + 2H2O + O2 → 4HNO3
Nitric acid obtained is concentrated above 50%. On further distillation, 68% nitric acid is produced.
Properties of Nitric Acid
- Physical Properties
Colour, odour and taste: Pure acid (98% conc.) is colourless, suffocating and sour to taste.
Physiological nature: Non-poisonous, highly corrosive
Density: Heavier than water, with specific gravity 1.54 g/cm3
Solubility: Highly soluble in water
Boiling point: 86°C
Freezing point: −42°C
- Chemical Properties
Uses of Nitric Acid
- To etch designs on copper and brassware because it acts as a solvent for several metals, except the noble metals
- To purify gold from impurities of Cu, Ag and Zn which dissolve in nitric acid
- As a rocket fuel oxidant
- In preparation of fertilisers such as Ca(NO3)2 and NH4NO3
- In preparation of aqua regia, which dissolves noble metals
Industrial Uses
In the manufacture of
- Explosives such as TNT
- Synthetic fertilisers such as artificial silk, nylon, plastics etc.
- Important compounds such as nitrates of potassium, ammonium, silver etc.
- Dyes, drugs and perfumes
Phosphorus – allotropic forms
Phosphorus is found in many allotropic forms, the important ones being white, red and black.
Introduction
White phosphorus
- It is a translucent white waxy solid. It is poisonous; it is soluble in carbon disulphide and glows in dark (chemiluminescence). It dissolves in boiling NaOH solution in an inert atmosphere giving PH3, but it is insoluble in water.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(sodium hypohoshite)
- White phosphorus having less stability because of that it is more reactive than the other solid phases under normal conditions because of angular strain in the P4 molecule where the angles are only 60°. It readily catches fire in air to give dense white fumes of P4O10.
P4 + 502 → P4O10
- It made up of individually separate tetrahedral P4molecule.
Red phosphorus
- It is obtained by heating white phosphorus at 573K in an inert atmosphere for several days.
- After the red phosphorus is heated under high pressure, a series of phases of black phosphorus are formed.
- Red phosphorus possesses iron grey lustre.
- It is odourless, nonpoisonous and insoluble in water as well as in carbon disulphide.
- Red phosphorus is polymeric, consisting of chains of P4 tetrahedral linked together in the manner.
Black phosphorus
- Black phosphorus has two forms α-black phosphorus and β-black phosphorus.
- α-Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803K. It can be sublimed in air and has opaque monoclinic or rhombohedral crystals. It does not oxidise in air.
- β-Black phosphorus is prepared by heating white phosphorus at 473 K under high pressure. It does not burn in air upto 673 K.
Preparation
- The reaction of calcium phosphide with water or dilute HCl forms Phosphine is prepared.
Ca3P2 + 6H2O → 3 Ca(OH)2 +2 PH3
Ca3P2 + 6HCI → 3 CaCI2 +2 PH3
- In the laboratory, it is prepared by heating white phosphorus with concentrated NaOH solution in an inert atmosphere of CO2.
P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(sodium hypohoshite)
- It is non inflammable when pure, but becomes inflammable owing to the presence of P2H4 or P4 vapours.
- To purify it from the impurities, it is absorbed in HI to form phosphonium iodide (PH4I) which on treating with KOH gives off phosphine.
PH4I+ KOH → KI + H2O + PH3
Properties
Physical properties
- It is a colourless gas with rotten fish smell and is highly poisonous.
- It explodes in contact with traces of oxidising agents like HNO3, Cl2 and Br2 vapours.
Chemical properties
- It is slightly soluble in water. The solution of PH3 in water decomposes in presence of light giving red phosphorus and H2.
- When absorbed in copper sulphate or mercuric chloride solution, the corresponding phosphides are obtained.
3CUSO4 + 2 PH3 → Cu3P2 +3H2SO4
3HgCI2 + 2 PH3 → Hg3P2 +6HCI - Phosphine is weakly basic and like ammonia, gives phosphonium compounds with acids.
e.g., PH3 + HBr → PH4Br
Uses
- The spontaneous combustion of phosphine is technically used in Holme’s signals.
- Containers containing calcium carbide and calcium phosphide are pierced and thrown in the sea when the gases evolved burn and serve as a signal.
- It is also used in smoke screens.
Sulphur and its compounds
Sulphur – allotropic forms
Sulphur forms variety of allotropes. The most common allotropes are yellow rhombic and monoclinic – sulphur. Rhombic sulphur is more stable at room temperature. It gets transforms to monoclinic sulphur when heated above 369 K.
Rhombic sulphur
- This allotrope is yellow in colour, m.p. is around 385.8 K and specific gravity is 2.06.
- Rhombic sulphur crystals are formed when the solution of roll sulphur in CS2 is evaporated.
- It is insoluble in water but dissolves to some extent in benzene, alcohol and ether. It is more soluble in CS2.
Monoclinic sulphur
- Its m.p. is 393 K and specific gravity 1.98. It is soluble in CS2.
- This form of sulphur is prepared by melting rhombic sulphur in a dish and cooling, till crust is formed.
- Two holes are made in the crust and the remaining liquid poured out. After removing crust, colourless needle shaped crystals of sulphur are formed.
- It is stable above 369 K and transforms into sulphur below 369 K.
- Also we can say that the sulphur is stable below 369 K and transforms into sulphur above this. At 369 K both the forms are stable. This temperature is called transition temperature.
- Rhombic as well as monoclinic sulphur have S8 molecules. These S8 molecules are packed to give different crystal structures. The S8 ring in both the forms is puckered and has a crown shape
- In cyclo-S6 form, the molecule is in chair shape.
- At very high temperatures (~1000 K); S2 is paramagnetic like O2.
Sulphur dioxide
Preparation
- It can be preapared in laboratory with action of metallic sulphate on a dilute acid.
Na2SO3 + H2SO4 → Na3SO4 +H2O + SO2
- Sulphur dioxide is formed together with a trace amount of sulphur trioxide (6-8%), when sulphur is burnt in air or oxygen:
S(s)+ O2(g) → SO2(g)
- In the laboratory, sulphite is treated with dilute sulphuric acid to give sulphurdioxide.
SO3 2- (aq) + 2H+ (aq) → H2O (I) + SO2 (g)
- It is also produced as a by-product of the roasting of sulphide ores.
4FeS2 (s) + 110 (g) → 2Fe2O3 (s) + 8 SO2 (g)
- The gas is first dried and is liquefied under pressure and stored in steel cylinders.
Properties
Physical Properties
- Sulphur dioxide is one of the gases that can be easily liquefied.
- Sulphur dioxide is a colourless gas with pungent smell and is highly soluble in water.
- It liquefies at room temperature under a pressure of 2 atmospheres and boils at 263 K.
- When Sulphur dioxide is passed through water, it forms a solution of sulphurous acid.
Chemical Properties
- It reacts with sodium hydroxide solution to give sodium sulphite, which then reacts with excess of sulphur dioxide to form sodium hydrogen sulphite.
2NaOH + SO2 → Na2SO3 + H2O
Na2SO3 + H2O + SO2 → 2NaHSO3
- When sulphur dioxide reacts with water or alkali, its behavior is similar to that of carbondioxide.
- Sulphur dioxide reacts with chlorine in the presence of charcoal (which acts as a catalyst) to give sulphuryl chloride, SO2Cl2.
- It is oxidised to sulphur trioxide by oxygen in the presence of vanadium (V) oxide catalyst.
SO2 (g) + Cl2 (g) → SO2Cl2(l)
2SO2 (g) + O2 (g) 3/4 1/4 → SO3 (g)
- Under moist conditions, sulphur dioxide behaves as a reducing agent. For example, it converts iron (III) ions to iron (II) ions and decolourises acidified potassium permanganate (VII) solution.
- The molecule of SO2 is angular. It is a resonance hybrid of the two canonical forms:
Uses
- It is used as a bleaching agent.
- In refining petroleum and sugar.
- In bleaching wool and silk.
- As an anti-chlor, disinfectant and preservative.
- Sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite (industrial chemicals) are manufactured from sulphur dioxide.
- Liquid SO2 is used as a solvent to dissolve a number of organic and inorganic chemicals.
Oxoacids of sulphur :
- Sulphur dioxide is a strong oxidizing agent.
- Sulphur forms a number of oxoacids such as H2SO3, H2S2O3, H2S2O4, H2S2O5, H2SxO6 (x = 2 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8.
- Some of these acids are unstable and cannot be isolated.
- They are commonly occurring in the form of aqueous solution or in the form of their salts.
Sulphuric Acid
Preparation
- Sulphuric acid is one of the most important industrial chemical.
- Sulphuric acid is manufactured by the Contact Process which involves three steps:
- Burning of sulphur or sulphide ores in air to generate SO2.
- Conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5).
- Absorption of SO3 in H2SO4 to give oleum (H2S2O7).
- A flow diagram for the manufacture of sulphuric acid.
- The SO2 produced by this process is purified by removing dust and other impurities such as arsenic compounds.
- The major step in the manufacture of H2SO4 is the catalytic oxidation of SO2 with O2 to give SO3 in the presence of V2O5 (catalyst).
2SO2 (g) + O2 (g) 3/4 1/4 → 2SO3 (g) D rHQ = -196.6 kJmol-1 - The reaction is exothermic, reversible. The forward reaction leads to a decrease in volume.
- Low temperature and high pressure are the favourable conditions for maximum yield.
- But the temperature should not be very low otherwise rate of reaction will become slow.
- In actual practice, the plant is operated at a pressure of 2 bar and a temperature of 720 K.
- The SO3 gas from the catalytic converter is absorbed in concentrated H2SO4 to produce oleum. Dilution of oleum with water gives H2SO4 of the required concentration.
- In the industry two steps are carried out simultaneously to make the process a continuous one and also to reduce the cost.
SO3 + H2SO4 → H2S2O7 - The sulphuric acid obtained by Contact process is 96 - 98% pure.
Properties
- Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of 1.84 at 298 K.
- The acid freezes at 283 K and boils at 611 K.
- It is highly exothermic in presence of water. It dissolves in water with the evolution of a large quantity of heat.
- Hence, care must be taken while preparing sulphuric acid solution from concentrated sulphuric acid.
- The concentrated acid must be added slowly into water with constant stirring.
- The chemical reactions of sulphuric acid are as a result of the following characteristics:
- In aqueous solution, sulphuric acid ionises in two steps.
H2SO4 (aq) + H2O(l) → H3O= (aq) + HSO4-(aq) Ka = very large (ka>10)
HSO4- (aq) + H2O(l) → H3O+ (aq) + SO42- (aq) ; Ka2 =1.2 10-2
- The larger value of K a1 (K a1 >10) means that H2SO4 is largely dissociated into H+ and HSO4.
- Greater the value of dissociation constant (Ka), the stronger is the acid.
- The acid forms two series of salts: normal sulphates (such as sodium sulphate and copper sulphate) and acid sulphates (e.g., sodium hydrogen sulphate).
- Sulphuric acid, because of its low volatility can be used to manufacture more volatile acids from their corresponding salts.
2MX+ H2SO4 → 2HX + M2SO4 (X=F,Cl,NO3)
- Concentrated sulphuric acid is a strong dehydrating agent.
- Many wet gases can be dried by passing them through sulphuric acid, provided the gases do not react with the acid.
- Sulphuric acid removes water from organic compounds; it is evident by its charring action on carbohydrates.
C12H22O11 HSO4 → 12 C + 11 H2O
- Hot concentrated sulphuric acid is a moderately strong oxidizing agent.
- In this respect, it is intermediate between phosphoric and nitric acids.
- Both metals and non-metals are oxidised by concentrated sulphuric acid, which is reduced to SO2.
Cu +2H2SO4 (conc.) → CuSO4 + SO2 +2H2O
S +2H2SO4 (conc.) → 3 SO2 +2H2O
C +2H2SO4 (conc.) → CO2 + 2 SO2 +2H2O
Uses
- Sulphuric acid is a very important industrial chemical as many other chemicals can be prepared from it.
- Primary use of sulphuric acid is in synthesis of fertilizers.
- The industrial strength can be judged by the quantity of sulphuric acid it produces and consumes.
- It is needed for the manufacture of hundreds of other compounds and also in many industrial processes.
- The bulk of sulphuric acid produced is used in the manufacture of fertilisers. (e.g., ammonium sulphate, superphosphate).

