Chemical Kinetics and Equilibrium
Chemical Kinetics and Equilibrium Synopsis
Synopsis
Rate of Chemical Reaction
Rate of Chemical Reaction:
- Alternately the rate of reaction can also be expressed as,
- Consider a hypothetical reaction, assuming that the volume of the system remains constant. R → P One mole of the reactant R produces one mole of the product P.
- If [R]1 and [P]1 are the concentrations of R and P respectively at time t1 and [R]2 and [P]2 are their concentrations at time t2 then,
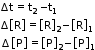
The square brackets in the above expressions are used to express molar concentration.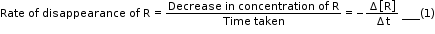
- Δ[R] is a negative quantity because concentration of reactants is decreasing.
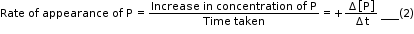
- Equations (1) and (2) given above represent the average rate of a reaction, rav.
This average rate depends upon the change in concentration of reactants or products and the time taken for that change to occur.
Units of rate of a reaction:
- From equations (1) and (2), it is clear that units of rate are concentration time–1.
- For example, if concentration is in mol L–1 and time is in seconds then the units will be mol L-1s–1.
- In gaseous reactions, the concentration of gases is expressed in terms of their partial pressures; hence the units of the rate equation will be atm s–1.
Instantaneous Rate of Reaction:
- Consider a hydrolysis of butyl chloride (C4H9Cl).
C4H9Cl+ H2O → C4H9OH + HCl - We have provided the concentrations over different intervals of time below.
- We can determine the difference in concentration over different intervals of time and thus determine the average rate by dividing Δ[R] by Δt.
- It can be seen from experimental data that the average rate falls from 1.90 × 10-4 mol L-1s-1 to 0.4 × 10-4 mol L-1s-1.
- However, average rate cannot be used to predict the rate of a reaction at a particular instant as it would be constant for the time interval for which it is calculated.
- Hence, to express the rate at a particular moment of time we determine the instantaneous rate.
- It is obtained when we consider the average rate at the smallest time interval say dt, when Δt approaches zero.
Therefore, for an infinitesimally small dt, instantaneous rate is given by,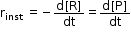
- By drawing the tangent at time t on the either of the curves for concentration of R vs time t or concentration of P vs time t and calculating the slope of the curve, we can determine the instantaneous rate of reaction.
- Hence, here in this example rinst at 600s is calculated by plotting graph of concentration of butyl chloride as against time t.
- A tangent is drawn on the curve at a point t = 600s.
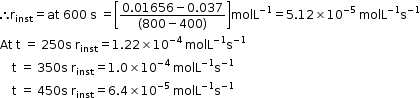
- Now consider a reaction,
Hg(l) +Cl2(g) → HgCl2 (s)
Here, the stoichiometric coefficients of the reactants and products are same; hence rate of the reaction is given as,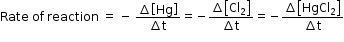
Therefore, we can say that from above equation that the rate of disappearance of any of the reactants is same as the rate of appearance of the products.
- Consider another reaction,
2HI(g) → H2(g) +I2(g)
In this reaction, two moles of HI decompose to produce one mole each of H2 and I2 i.e. the stoichiometric coefficients of reactants or products are not equal to one; hence we need to divide the rate of disappearance of any of the reactants or the rate of appearance of products by their respective stoichiometric coefficients.
Since rate of consumption of HI is twice the rate of formation of H2 or I2, to make them equal, the term Δ[HI] is divided by 2.
The rate of this reaction is given by,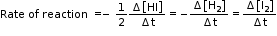
- For a gaseous reaction at constant temperature, concentration is directly proportional to the partial pressure of a species and hence, rate can be expressed as rate of change in partial pressure of the reactant or the product.
Factors affecting the rate of reaction:
EQUILIBRIA IN CHEMICAL PROCESS
Irreversible Reactions
Irreversible reactions: The chemical reactions which proceed in such a way that reactants are completely converted into products, i.e. the reactions which move in one direction, i.e. forward direction only are called irreversible reactions.
(a) (i) Thermal decomposition of ammonium nitrite,
NH4NO2 → N2+2H2O
(b) Precipitation reaction,
(ii) AgNO3+ NaCl → AgCl +NaNO3
(iii) Pb(NO3)2 +2KI → PbI2 + 2KNO3
(c) Neutralisation reactions:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Strong Acid Strong Base
Reversible Reactions:
Reactions which thus proceed in both the directions and do not reach to completion are known as reversible reactions. The reaction proceeding from left to right is conventionally called the forward reaction and the opposite one proceeding from right to left is called the reverse or backward reaction. In such reactions the arrow (→ ) or sign of equality (=) is replaced by two half arrow ( ⇌ ) pointing the reaction in both the directions. This sign (⇌) represents the reversibility of the reaction
3Fe +4H2O ⇌ Fe3O4 + 4H2
Some examples of reversible reactions are given below:
CaCO3 ⇌ CaO + CO2
CH3COOH + C2H5OH ⇌ CH3COOC2H5 +H2O
Heterogeneous Reactions: The reversible reaction in which more than one phase is present is called heterogeneous reaction.
CaCO3(s) ⇌ CaO(s) +CO2(g)
MgCO3(s) ⇌ MgO(s) + CO2(g)
2Na2O2(s)+ 2H2O(𝓁) ⇌ 4NaOH(𝓁) +O2(g)
3Fe(s) +4H2O(𝓁)⇌ Fe3O3 (s) +4H2(g)
- A system needs to be always closed to achieve equilibrium since an open system allows the escape of the formed products which prevents the backward reaction.
- Chemical equilibrium, at a given temperature, is characterized by constancy of certain properties such as pressure, concentration, density or colour.
- Chemical equilibrium can be attained from either side, i.e., from the side of reactants or products.
N2 + 3H2 ⇌ 2NH3 - Aequilibrium, each reactant and each product has a fixed concentration and this is independent of the fact whether we start the reaction with the reactants or with the products. This reaction can be graphically represented as,
- resence of catalyst never affects the equilibrium but it helps in attaining it rapidly.
- The reactions move with the same speed exhibiting the dynamic nature
- Rate of forward reaction = rate of backward reaction
- Concentration (mole/litre) of reactant and product becomes constant.
- ΔG = 0
- Q = Keq
- Stoichiometric representation of a reaction
- Mode of representing the change
- Temperature
- Units of pressure
- Units of Kp = (unit of pressure)∆n
- Units of Kc = (unit of concentration)∆n
- If Q = K, the reaction is in equilibrium.
- If Q > K, the reaction moves from right to left.
- If Q < K, the reaction moves from left to right.
- If the concentration of reactant is increased at equilibrium, then the reaction shifts in the forward direction.
- If the concentration of product is increased, then the equilibrium shifts in the backward direction.
- If the volume is increased, the pressure decreases; hence, the reaction will shift in the direction in which pressure increases,
i.e. in the direction in which the number of moles of gases increases and vice versa. - If the volume is increased, then for
Δn > 0, the reaction will shift in the forward direction
Δn < 0, the reaction will shift in the backward direction
Δn = 0, the reaction will not shift
- Constant pressure:
If the inert gas is added, then to maintain the pressure constant, volume is increased. Hence, the equilibrium will shift in the
direction in which larger number of moles of gas is formed.
Δn > 0, the reaction will shift in the forward direction
Δn < 0, the reaction will shift in the backward direction
Δn = 0, the reaction will not shift - Constant volume:
Inert gas addition has no effect at constant volume.
Equilibrium constant is only dependent on the temperature.

- For an endothermic (ΔH > 0) reaction, the value of the equilibrium constant increases with increase in temperature.
- For an exothermic (ΔH < 0) reaction, the value of the equilibrium constant decreases with increase in temperature.
- For ΔH > 0, the reaction shifts in the forward direction with increase in temperature.
- For ΔH < 0, the reaction shifts in the backward direction with increase in temperature.
- If the concentration of reactant is increased at equilibrium, then the reaction shifts in the forward direction.
- If the concentration of product is increased, then equilibrium shifts in the backward direction.

