Organic Chemistry - II
Organic Chemistry -II Synopsis
Synopsis
Alcohols
- Alcohols are hydroxyl derivatives of alkanes obtained by the replacement of one, two or three hydrogen atoms of alkanes by the corresponding number of –OH groups.
- The hydroxyl group is the functional group of alcohols.
- The general molecular formula of alcohols is CnH2n+1 OH.
Sources
- Ethanol is obtained from the fermentation of sugars, while methanol is obtained from the destructive distillation of wood.
- Cracking of petroleum is a source of ethane which is used for preparing ethanol.
- Preparation of Ethanol
- Laboratory preparation by hydrolysis of alkyl halides
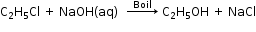
- Industrial Method
- Hydration of Ethene
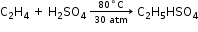
C2H5HSO4 + H2O →C2H5OH + H2SO4 - Fermentation of Carbohydrates
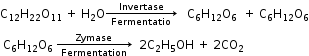
Properties of Alcohols
- Physical properties
- Inflammable volatile liquid.
- The boiling point increases with an increase in the molecular weight.
Example: Methanol = 64.5°C and ethanol = 78.3°C
- Soluble in water and organic solvents.
- Ethanol is lighter than water with a density of 0.79 cm−1 at 293 K.
- Chemical properties
- Combustion (burning):
C2H5OH + 3O2 → 2CO2 + 3H2O - Oxidation with K2Cr2O7
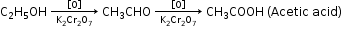
- Action with Sodium
2C2H5OH + 2Na →2C2H5ONa + H2
(Sodium ethoxide) - Action with Acetic Acid
C2H5OH + CH3COOH → CH3COOC2H5 + H2O
(Ethyl acetate)5. - Action with Sulphuric Acid
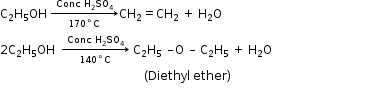
- Action with PCl3
3C2H5OH + PCl3 → 3C2H5Cl + H3PO3
Uses of Alcohol
- As a solvent for gums and resins
- Used in thermometers because of its low freezing point
- In alcoholic drinks
- In the manufacture of chemicals and synthetic products such as dyes, perfumes, antiseptics, preservatives etc.
Carboxylic Acids
- Carboxylic acids are organic compounds containing a carboxylic group (–COOH) attached to an alkyl group or to a hydrogen atom.
- Representation of carboxylic acids: R-COOH [R is either –H or alkyl]
- The functional group of carboxylic acids: –COOH [carboxylic]
- The acidic character in carboxylic acids is because of the presence of the replaceable hydrogen atom in the carboxylic group.
Occurrence
Acids occur in the free state in many fruits and as esters in several essential oils.
- Preparation of Acetic Acid
- By oxidation of ethyl alcohol
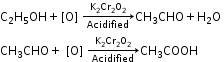
- By hydrolysis of ethyl acetate
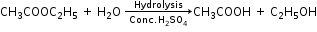
Properties
Physical properties
- It is a colourless liquid with a pungent smell.
- Boiling point is 118°C, and melting point is 17°C.
- It is a hygroscopic liquid; specific gravity at 0°C is 1.08.
- Miscible in water, alcohol and ether in all proportions.
- Chemical properties
- It is a weak acid and turns blue litmus red.
2CH3COOH + Zn → (CH3COO)2Zn + H2 ↑ - Reaction with Alkalis
CH3COOH + NaOH → CH3COONa + H2O
CH3COOH + NH4OH → CH3COONH4 + H2O - Reaction with Carbonates
2CH3COOH + Na2CO3 →2CH3COONa + H2O + CO2
CH3COOH + NaHCO3→CH3COONa + H2O + CO2 - Reaction with Alcohols
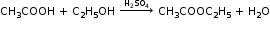
- Reaction with PCl3
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl - Reduction
CH3COOH + 4[H] →C2H5OH + H2O
Uses of Acetic Acid
- As a solvent for resins, cellulose etc.
- As a laboratory reagent
- As vinegar
- In medicines
- For coagulating rubber from latex
Preparation of Aldehydes and Ketones
- Oxidation of Alcohols
Aldehydes and ketones can be prepared by oxidation of primary and secondary alcohols.
- Dehydrogenation of Alcohols
On passing vapours of alcohol over metal catalysts like Cu at 573K, dehydrogenation takes place.
Primary alcohols give aldehydes whereas secondary alcohols yield ketones. - From Hydrocarbons
By ozonolysis of Alkenes
Alkenes on ozonolysis followed by reaction with zinc dust and water yields aldehydes and ketones. - By Hydration of Alkynes
Alkynes undergo addition reaction with water in the presence of got dil. H2SO4 and HgSO4 to give aldehydes or ketones.
Preparation for Aldehydes - Rosenmund Reduction
In this reaction, acyl chloride on hydrogenation in the presence of palladium catalyst and barium sulphate gives aldehydes. - Stephen Reaction
Nitriles on reduction with stannous chloride in the presence of HCl give imine which on hydrolysis gives corresponding aldehyde.
An alternate method to reduce nitriles selectively is by diisobutylaluminium hydride to imines which on hydrolysis yields aldehydes.
Esters can also be reduced to aldehydes with DIBAL-H - From Aromatic Hydrocarbons
Aromatic aldehydes can be prepared using the following methods.
- IBy Oxidation of Methylbenzene
Etard Reaction ( Use of Chromyl Chloride)
Chromyl chloride oxidises the methyl group to a chromium complex which on further gives corresponding benzaldehyde.
Use of Chromic oxide(CrO3)
Toluene when treated with chromic oxide in acetic anhydride gets converted into benzylidene diacetate which on hydrolysis with aqueous acid gives benzaldehyde. - Side Chain Chlorination
Toluene on side chlorination gives benzal chloride which on hydrolysis gives benzaldehyde. - Gatterman –Koch Reaction
Benzene or toluene on treatment with CO and HCl in the presence of AlCl3 or CuCl gives benzaldehyde or p-tolualdehyde.
Preparation for Ketones
- From Acid chlorides or Acyl chlorides
Acyl chloride on treatment with dialkylcadmium obtained by reaction of cadium chloride with Grignard reagent gives ketones.
Example:
- From Nitriles
Nitriles on treatment with Grignard reagent followed by hydrolysis yields a ketone. - From Benzene or Substituted Benzenes
Benzene or substituted benzene on treatment with acid chloride in the presence of anhydrous AlCl3 gives the corresponding ketone and this reaction is known as Friedel-Crafts acylation reaction.
Physical Properties
Boiling Points
- The boiling points of aldehydes and ketones are higher than those of hydrocarbons and ethers of comparable molecular masses.
- This is because the carbonyl group in aldehydes and ketones is polar and hence show weak intermolecular association due to dipole-dipole interactions between the opposite ends of the
dipoles.
- They have lower boiling points than those of alcohols of similar masses due to absence of intermolecular hydrogen bonding.
Solubility
- The lower members of aldehydes and ketones such as methanol, ethanol and propanone are miscible in water because they form hydrogen bond with water.
- The solubility decreases on increasing the length of alkyl chain.
- All aldehydes and ketones are fairly soluble in organic solvents like benzene, ether, methanol etc.
Chemical Reactions
- Nucleophilic Addition Reactions
Aldehydes and Ketones undergo nucleophilic addition reactions.
(i)Mechanism for Nucleophilic Addition Reactions
- A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group perpendicularly to the sp3 hybridised orbitals of carbonyl carbon.
- The hybridisation changes from sp2 to sp3 and a tetrahedral alkoxide intermediate is formed.
- The intermediate grabs a proton from the reaction medium to give an electrically neutral product.
- The net result is addition of Nu- and H+ across the C-O double bond.
(ii)Reactivity
- Aldehydes are more reactive than ketones in nucleophilic reactions because of two reasons:
- Sterically, it is the presence of two relatively large groups in ketones that hinder the approach of nucleophile to carbonyl carbon than in aldehydes which have only one such substituent.
- Electronically, aldehydes are more reactive than ketones because the two alkyl groups in ketones decrease the electrophilicity of the carbonyl carbon more effectively than in aldehydes.
(iii)Important Examples of Nucleophilic Addition and Nucleophilic Addition- Elimination Reactions
(a)Addition of Hydrogen cyanide(HCN)
- On addition of HCN to aldehydes and ketones they yield cyanohydrins.
- Since the reaction is very slow with pure HCN, it is catalysed with the help of a base and the cyanide ion (CN-) generated as a strong nucleophile adds to carbonyl compounds to give cyanohydrins.
(b)Addition of Sodium Hydrogensulphite
- Sodium hydrogen sulphite when added to aldehydes and ketones yield addition products.
- For most aldehydes the equilibrium is on the right hand side and for most ketones it is on the left hand side due to steric factors.
(c)Addition of Grignard Reagents
Grignard reagents on reacting with aldehydes and ketones yield alcohols.
For example:
- Methanal produces primary alcohol with Grignard reagent.
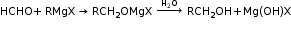
- Aldehydes produce secondary alcohols with Grignard reagent.
- Ketones produce tertiary alcohols with Grignard reagent.
(d)Addition of Alcohols
- Aldehydes on treatment with one equivalent of monohydric alcohol in the presence of dry HCl give hemiacetal which on further treatment with one more molecule of alcohol gives acetal.
- Ketones also react with ethylene glycols under similar conditions to give ethylene glycol ketals.
- The role of dry HCl is to protonate the oxygen of carbonyl compounds and thereby increasing the electrophilicity of the carbonyl carbon towards nucleophilic addition of ethylene glycol.
(e)Addition of Ammonia and its Derivatives
- Ammonia and its derivative add to the carbonyl group of an aldehydes and ketones.
- The reaction is reversible and acid catalysed and favours the product formation due to the rapid dehydration of the intermediate to form >C=N-Z.
- Reduction
- Reduction to Alcohols
Aldehydes and ketones get reduced to primary and secondary alcohols by NaBH4 or LiAlH4. - Reduction to Hydrocarbons
Aldehydes and ketones reduce to –CH2 group on treatment with zinc-amalgam and conc. HCl[Clemmenson reduction] or with hydrazine which on heating with sodium or potassium hydroxide in ethylene glycol[Wolff-Kishner reduction].
Oxidation
- Aldehydes get oxidised to carboxylic acids with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate etc.
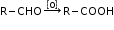
- Ketones undergo oxidation with strong oxidising agents and elevated temperatures. The reaction involves carbon-carbon bond cleavage to give a mixture of carboxylic acids with lesser number of carbon atoms than the parent ketones.
Carbohydrates
Carbohydrates and Their Classification
Carbohydrates form a very large group of naturally occurring organic compounds which play a vital role in daily life. They are produced in plants by the process of photosynthesis. The most common carbohydrates are glucose, fructose, sucrose, starch, cellulose etc. Carbohydrates may be defined as optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis.
The most common sugar, used in our homes is named as sucrose whereas the sugar present in milk is known as lactose. Depending upon their behaviour on hydrolysis carbohydrates are classified into three groups.
Classification of Carbohydrates
- Monosaccharides:
A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide. About 20 monosaccharides are known to occur in nature. Glucose, fructose, ribose are most common examples. - Oligosaccharides:
Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides. They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc., depending upon the number of monosaccharides, they provide on hydrolysis. Sucrose, Lactose, Maltose are most common examples. - Polysaccharides:
Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides. Starch, cellulose, glycogen, gums are most common examples. Polysaccharides are not sweet in taste; hence they are also called non-sugars.
The carbohydrates may also be classified as either reducing or non-reducing sugars. All those carbohydrates which reduce Fehling’s solution and Tollen’s’ reagent are referred to as reducing sugars. All monosaccharides whether aldose or ketose are reducing sugars. In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars e.g. sucrose.
Monosaccharides
Monosaccharides are further classified on the basis of the number of carbon atoms and the functional group present in them. If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose.
Glucose (Aldohexose)
Glucose is the monomer for many other carbohydrates. Alone or in combination, glucose is probably the most abundant organic compound on the earth. Glucose occurs freely in nature as well as in the combined form. It is present in sweet fruits and honey.
Classification of Polymers
Introduction
- Polyme
The word ‘polymer’ is coined from two Greek words.
They are very large molecules having high molecular mass (103-107u).
They are also called as macromolecules which are formed by joining of repeating structural units on a large scale. - Monomer
The repeating structural units which are derived from some simple and reactive molecules are known as monomers.
They are linked to each other by covalent bonds. - Polymerisation
The process of formation of polymers from respective monomers is called polymerisation.
Examples:
- Conversion of ethene to polyethene
- Formation of Nylon 6, 6 from the interaction of hexamethylene diamine and adipic acid.
Classification of Polymers
Based on some special considerations following are some of the common classifications of polymers:
Classification Based on Source
In this type of classification, there are three sub categories:
- Natural Polymers
Proteins, cellulose, starch, some resins and rubber are examples of polymers found in plants. - Semi-synthetic Polymers
Cellulose derivatives like cellulose acetate (rayon) and cellulose nitrate etc. are examples of this category. - Synthetic Polymers
Plastic (polythene), nylon (6, 6) and synthetic rubbers (Buna –S) are examples of man-made polymers.
Classificatin Based on Structure of Polymers
- Linear Polymers
These polymers are made up of long and straight chains.
Examples: High density polythene, polyvinyl chloride - Branched Chain Polymers
They contain linear chains having some branches.
Example: Low density polythene - Cross linked or Network Polymers
It is formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains.
Example: Bakelite, melamine etc.
Classification Based on Mode of Polymerisation
Polymers can also be classified on the basis of mode of polymerisation into two sub groups.
- Addition Polymers
It is formed by the repeated addition of monomer molecules possessing double or triple bonds.
The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers.
Example: Formation of polythene from ethene.
The addition polymers formed by the polymerisation of two different monomers are known as copolymers.
Example: Buna-S, Buna-N, etc. - Condensation Polymers
- These polymers are formed by repeated condensation reaction between two different bi-functional or tri-functional monomeric units.
- In these reactions, elimination of small molecules such as water, alcohol, hydrogen chloride etc. takes place.
Classification Based on Molecular Forces
- Polymers find applications in various fields depending on their unique mechanical properties like tensile strength, elasticity, toughness etc.
- These mechanical properties are governed by intermolecular forces like van der Waals forces and hydrogen bonds present in the polymer.
- In this category, polymers are classified into four sub groups on the basis of magnitude of intermolecular forces present in them.
1. Elastomers
- These are rubber solids with elastic properties.
- In these elastomeric polymers, the polymer chains are held together by weakest intermolecular forces which allow the polymer to be stretched.
- A few ‘crosslinks’ are introduced in between the chains which help the polymer to retract to its original position after the force is released as in vulcanised rubber.
Example: Buna-S, Buna-N, neoprene etc.
2.Fibres
- They are thread forming solids with high tensile strength and high modulus.
- These characteristics are because of the strong intermolecular forces like hydrogen bonding.
- These strong forces also lead to close packing of chains and thus impart crystalline nature.
Example: Polyamides (nylon 6, 6), polyesters (terylene), etc.
3. Thermoplastic polymers
- These are the linear or slightly branched long chain molecules which soften on heating and harden on cooling.
- These polymers have intermediate forces of attraction intermediate between elastomers and fibres.
Example: polythene, polystyrene, polyvinyls, etc.
4. Thermosetting polymers
They are cross linked or heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible and cannot be reused.
5. Example: Bakelite, urea-formaldehyde resins etc.
Polymers of Commercial Importance
TOP Concepts
- Drugs: Drugs are low molecular mass ( 100–500 u) substances which interact with targets in the body and produce a biological response.
- Medicines: Medicines are chemicals which are useful in diagnosis, prevention and treatment of diseases.
- Therapeutic effect: Desirable or beneficial effect of a drug such as treatment of symptoms and cure of a disease is known as therapeutic effect.
- Enzymes: Proteins which perform the role of biological catalysts in the body are called enzymes.
- Functions of enzymes
- The first function of an enzyme is to hold the substrate for a chemical reaction. Active sites of enzymes hold the substrate molecule in a suitable position so that it can be attacked by the reagent effectively.
- The second function of an enzyme is to provide functional groups which will attack the substrate and carry out the chemical reaction.
- The main role of drugs is to either increase or decrease the role of enzyme-catalysed reactions. Inhibition of enzymes is a common role of drug action. An enzyme inhibitor is a drug which inhibits the catalytic activity of enzymes or blocks the binding site of the enzyme and eventually prevents the binding of the substrate with the enzyme. Drugs can inhibit the attachment of the substrate to the active site of enzymes in the following ways.
- Competitive Inhibition: Competitive inhibitors are the drugs which compete with the natural substrate for their attachment on the active sites of enzymes.
- Non-competitive Inhibition: Some drugs do not bind to the enzyme’s active site but instead bind to a different site of the enzyme called the allosteric site. This binding of the inhibitor at the allosteric site changes the shape of the active site in such a way that the substrate cannot recognise it. If the bond formed between an enzyme and an inhibitor is a strong covalent bond and cannot be broken easily, then the enzyme is blocked permanently. The body then degrades the enzyme–inhibitor complex and synthesises the new enzyme.
- Receptors as Drug Targets
Proteins which are vital for the communication system in the body are called receptors. In the body, the message between two neurons and that between neurons to muscles is communicated through chemical messengers. They are received at the binding sites of receptor proteins. To accommodate a messenger, the shape of the receptor site changes which brings about the transfer of message into the cell. A chemical messenger gives the message to the cell without entering the cell.
Receptors show selectivity for one chemical messenger over the other because their binding sites have different shape, structure and amino acid composition.
Drugs which bind to the receptor site and inhibit its natural function are called antagonists. These are useful when the blocking of the message is required. Drugs which mimic the natural messenger by switching on the receptor are called agonists. These are useful when there is a lack of natural chemical messengers.
- Therapeutic action of different classes of drugs
- Antacid: Chemical substances which neutralise excess acid in the gastric juices and give relief from acid indigestion, acidity, heart burns and gastric ulcers. Examples: Eno, Gelusil, Digene etc.
- Antihistamines: Chemical substances which diminish or abolish the effects of histamine released in the body and hence prevent allergic reactions. Examples: Brompheniramine (Dimetapp) and terfenadine (Seldane)
- Neurologically Active Drugs: Drugs which have a neurological effect, i.e. affects the message transfer mechanism from nerve to receptor.
- Tranquillisers: Chemical substances used for the treatment of stress and mild or severe mental diseases. Examples: Derivatives of barbituric acids such as veronal, amytal, nembutal, luminal and seconal
- Analgesics: Chemical substances used to relieve pain without causing any disturbances in the nervous system such as impairment of consciousness, mental confusion, incoordination, paralysis etc.
- Classification of Analgesics
- Antimicrobials: Drugs which tend to destroy/prevent development or inhibit the pathogenic action of microbes such as bacteria (antibacterial drugs), fungi (antifungal agents), virus (antiviral agents) or other parasites (antiparasitic drugs) selectively.
- Antibiotics: Chemical substances produced by microorganisms which kill or prevent the growth of other microbes.
Classification of antibiotics on the basis of mode of control of microbial diseases:
Classification of antibiotics on the basis of its spectrum of action:
- Antiseptics: Chemical substances which kill or prevent the growth of microorganisms and can be applied on living tissues such as cuts, wounds etc. Examples: Soframycin, Dettol
- Disinfectants: Chemical substances which kill microorganisms but cannot be applied on living tissues such as cuts, wounds etc. Examples: Chlorine (Cl2), bithionol, iodoform etc.
- Antifertility drugs: Chemical substances used to prevent conception or fertilisation. Examples: Norethindrone, ethinyl estradiol (Novestrol)
- Food additives: Food additives are the substances added to food to preserve its flavour or improve its taste and appearance.
Different types of food additives:
- Soaps
- Soap: It is a sodium or potassium salt of long chain fatty acids such as stearic, oleic and palmitic acids.
- Saponification: The process of making soap by hydrolysis of fats or oils with alkalies.
- Types of soaps
- Advantages of using soaps: Soap is a good cleansing agent and is 100% biodegradable, i.e. microorganisms present in sewage water can completely oxidise soap. Therefore, soaps do not cause any pollution problems.
- Disadvantages of using soaps:
- Soaps cannot be used in hard water because hard water contains metal ions such as Ca2+ and Mg2+ which react with soap to form white precipitate of calcium and magnesium salts.
These precipitates stick to the fibres of the cloth as gummy mass and block the ability of soaps to remove oil and grease from fabrics. Therefore, it interferes with the cleansing ability of the soap and makes the cleansing process difficult.
- In acidic medium, the acid present in solution precipitate the insoluble free fatty acids which adhere to the fabrics and hence block the ability of soaps to remove oil and grease from the fabrics. Hence, soaps cannot be used in acidic medium.
- Detergents
- Detergents are sodium salts of long chain of alkyl benzene sulphonic acids or sodium salts of long chain of alkyl hydrogen sulphates.
Classification of detergents
- Anionic detergents:
Anionic detergents are sodium salts of sulphonated long chain alcohols or hydrocarbons. Alkyl hydrogen sulphates formed by treating long chain alcohols with concentrated sulphuric acid are neutralised with alkali to form anionic detergents. Similarly, alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali.
Example:
Anionic detergents are termed so because a large part of molecule is an anion.
Uses: They are used in household cleaning such as dishwasher liquids, laundry liquid detergents, laundry powdered detergents etc. Advantage: They are effective in slightly acidic solutions where soaps do not work efficiently.
- Cationic detergents: Cationic detergents are quaternary ammonium salts of amines with acetates, chlorides or bromides as anions. Cationic parts possess a long hydrocarbon chain and a positive charge on nitrogen atom.
Example:
Cationic detergents are termed so because a large part of the molecule is a cation.
Use: Because cationic detergents possess germicidal properties, they are used as germicides.
Advantage: They have strong germicidal action.
Disadvantage: These detergents are expensive.
- Non-ionic detergents: They do not contain any ion in their constitution. They are like esters of high molecular mass.
Example: The detergent formed by condensation reaction between stearic acid and polyethyleneglycol.
Use: In making liquid washing detergents.
Advantage: They have effective H-bonding groups at one end of the alkyl chain which make them freely water soluble.
- Biodegradable detergents: Detergents with straight hydrocarbon chains which are easily decomposed by microorganisms.
Example: Sodium lauryl sulphate - Non-biodegradable detergents: Detergents with branched hydrocarbon chains which are not easily decomposed by microorganisms.
Download complete content for FREE 

