Electrochemistry
Electrochemistry Synopsis
Synopsis
Basic Concept of Electrolysis:
- In an electrolytic cell external source of voltage is used to bring about a chemical reaction.
- One of the simplest electrolytic cell consists of two copper strips dipping in an aqueous solution of copper sulphate. If a DC voltage is applied to the two electrodes, then Cu2+ ions deposit at the cathode (negatively charged) and the following reaction takes place:
Cu2+ (aq) + 2e- → Cu(s)
At the anode, copper is converted into Cu2+ ions by the reaction:
Cu (s) → Cu2+(s) + 2e-
Thus copper is dissolved (oxidized) at anode and deposited (reduced) at cathode.
- This is the basis for an industrial process in which impure copper is converted into copper of high purity. This process is known as Electrolysis.
Faraday’s Laws of Electrolysis:
- Michael Faraday was the first scientist who described the quantitative aspects of electrolysis.
- After his extensive investigations on electrolysis of solutions and melts of electrolytes, Faraday published his results during 1833-34 in the form of the following well known Faraday’s two laws of electrolysis.
What is One Faraday?
- If the current ‘I’ is passed through an electrode to the electrolytic solution for a time ‘t’ then the quantity of electricity, Q can be given as:
Q = It
Where, ‘Q’ is in coulombs, ‘I’ is in ampere and‘t’ is in second. - The amount of electricity (or charge) required for oxidation or reduction depends on the stoichiometry of the electrode reaction. For example, in the reaction:
Ag+(aq)+e-→ Ag(s)
One mole of the electron is required for the reduction of one mole of silver ions. - We know that charge on one electron is equal to 1.6021 × 10–19C.
Therefore, the charge on one mole of electrons is equal to:
C=NA× 1.6021×10-19
= 6.02× 1023 mol-1 × 1.6021 ×10-19
∴ C =96487 c mol-1
This quantity of electricity is called Faraday and is represented by the symbol F.
For approximate calculations we use
1F = 96487 C mol-1 = 96500 C mol-1
Products of Electrolysis:
- For example, if we use molten NaCl, the products of electrolysis are sodium metal and Cl2 gas.
- Here we have only one cation (Na+) which is reduced at the cathode and one anion (Cl–) which is oxidized at the anode.
- During the electrolysis of aqueous sodium chloride solution, the products are NaOH, Cl2 and H2. In this case besides Na+ and Cl– ions we also have H+ and OH– ions along with the solvent molecules, H2O.
- Hence, the net reactions may be summarized as:
- The standard electrode potentials are replaced by electrode potentials given by Nernst equation to take into account the concentration effects.
Galvanic Cell:
- In this device the Gibbs energy of the spontaneous redox reaction is converted into electrical work which may be used for running motor or other electrical gadgets like heater, fan, geyser, etc.
- The following redox reaction occurs in Galvanic cell,
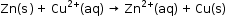
- This reaction is a combination of two half reactions whose addition gives the overall cell reaction:
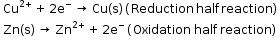
- The reduction half reaction occurs on the copper electrode while the oxidation half reaction occurs on the zinc electrode. These two portions of the cell are also called half-cells or redox couples.
- Diagram:
- Working:
- At each electrode-electrolyte interface there is a tendency of metal ions from the solution to deposit itself on the metal electrode and trying to make it positively charged.
- At the same time, metal atoms of the electrode have a tendency to go into the solution in the form of ions and leave behind the electrons at the electrode surface to make it negatively charged.
- At equilibrium, there is a separation of charges and depending on the tendencies of the two opposing reactions, the electrode may be positively or negatively charged with respect to the solution.
- A potential difference develops between the electrode and the electrolyte which is called electrode potential.
- The potential difference between the two electrodes of a galvanic cell is called the cell potential and is measured in volts.
- It is now an accepted convention that we keep the anode on the left and the cathode on the right while representing the galvanic cell.
- Under this convention the emf of the cell is positive and is given by
ECell=ERHS - ELHS
- Representation of Galvanic Cell:
- By convention, the electrode on which oxidation takes place is written on the left hand side and the other electrode on which reduction takes place is written on the right hand side.
- The electrode on LHS is written by writing the symbol of metal or the gas first, followed by the symbol of ion with its concentration in bracket.
- The electrode on RHS is written by writing first the ion along with its concentration in bracket followed by the symbol of the metal or gas.
- Single vertical lines represent the phase boundaries of the electrodes that are boundaries between metal and electrolyte solution.
- Double vertical lines represent the electrolytes connected by salt bridge.
- Consider an example of copper and silver electrode, where silver electrode acts as a cathode and copper electrode acts as an anode.
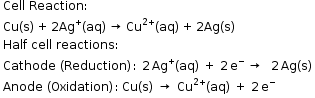
- The cell can be represented as:
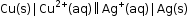
Stanard Electrode Potential:
Standard Hydrogen Electrode (SHE):
- The potential of individual half-cell cannot be measured. We can measure only the difference between the two half-cell potentials that gives the emf of the cell.
- If we arbitrarily choose the potential of one electrode (half cell) then that of the other can be determined with respect to this.
- According to convention, a half-cell called standard hydrogen electrode represented by
Pt(s) ⎥ H2(g) ⎥ H+(aq)
is assigned a zero potential at all temperatures corresponding to the reaction,
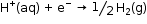
- Diagram:
Standard Hydrogen Electrode (SHE)
- Construction and Working:
- The standard hydrogen electrode consists of a platinum electrode coated with platinum black.
- The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it.
- The concentration of both the reduced and oxidized forms of hydrogen is maintained at unity. This implies that the pressure of hydrogen gas is one bar and the concentration of hydrogen ion in the solution is one molar.
- When the Standard Hydrogen Electrode is in contact with the second half cell, which is constructed by taking the SHE as anode (reference half cell) and other half cell as a cathode, it gives the emf of the cell as reduction potential of the other half cell at 298 K.
- If the concentrations of the oxidized and the reduced forms of the species in the right hand half-cell are unity, then the cell potential is equal to standard electrode potential,
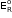 of the given half-cell.
of the given half-cell.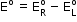
- As
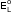 for standard hydrogen electrode is zero.
for standard hydrogen electrode is zero.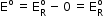
- The measured emf of the cell :
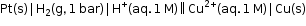
is 0.34 V and it is also the value for the standard electrode potential of the half-cell corresponding to the reaction: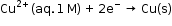
- Similarly, the measured emf of the cell :
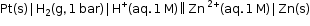
is -0.76 V corresponding to the standard electrode potential of the half-cell reaction: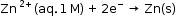
Electrochemical Series
It is a series in which metals are arranged based on the ease with which atoms of metals lose electrons to form positively charged ions.
Selective Discharge of Ions
The preferential discharge of ions present in an electrolyte at the respective electrodes is known as selective discharge of ions.
It depends on the following factors:
- Relative position of ions in an electrochemical series
- Concentration of the ions
- Nature of the electrode
Electrolysis
- Electrolysis of Fused Lead Bromide
Electrolyte: Molten lead bromide (PbBr2)
- Electrodes:
Anode: Graphite
Cathode: Graphite
Ions present:
Pb2+and Br–Overall reaction: PbBr2 →Pb2+ + 2Br–
Electrode reactions:
Reaction at the cathode: Pb2+ + 2e−→Pb
Reaction at the anode: Br− − e− →Br
Br + Br → Br2
- Electrolysis of Acidified Water
Electrolyte: Acidified water
Electrodes:
Anode: Platinum
Cathode: Platinum
Ionisation of acidified water: H2O ⇌ H+ + OH–
H2SO4 ⇌ 2H+ + SO42–
Ions present: H+, SO42–, OH–
Electrode reactions:
Reaction at the cathode: H+ + e−→ H
H + H → H2 (hydrogen molecule)
Reaction at the anode: OH– - e−→ OH × 4
OH– ion discharge in preference to SO42–
4OH → 2H2O + O2 (oxygen molecule)
- Electrolysis of Acidified Copper Sulphate – Using Platinum Anode and Copper or Platinum Cathode
Electrolyte: Acidified copper sulphate
Electrodes:
Cathode: Copper or platinum
Anode: Platinum
Ions present: Cu2+, H+, SO42–, OH– and H2O molecules
Dissociation of acidified copper sulphate:
CuSO4 ⇌ Cu2+ + SO42–
H2O ⇌ H+ + OH–
Electrode Reactions:
Reaction at the cathode: Cu2+ + 2e− → Cu
Reaction at the anode: 4OH − 4e− → 4OH
2OH + 2OH → 2H2O + O2
- Electrolysis of Aqueous Copper Sulphate – Using Copper Electrodes
Electrolyte: Aqueous copper sulphate solution
Electrodes:
Anode: Copper
Cathode: Copper
Ions present: Cu2+, SO42–, H+, OH–
Dissociation of aqueous copper sulphate:
CuSO4 ⇌ Cu2+ + SO42–
H2O ⇌ H+ + OH–
Electrode reactions:
Reaction at the cathode: Cu2+ + 2e− →Cu
Cu being lower in the electrochemical series
Reaction at the anode: Cu − 2e–→Cu2+
SO42– and OH– are not discharged.
- Applications of Electrolysis
- Electroplating with metals
- Electrorefining of metals
- Extraction of metals
Electroplating
It is a process in which a thin film of a metal, such as gold, silver or nickel, gets deposited on another metallic article with the help of electricity.
- Reasons for Electroplating
- Decoration purposes
- To protect from rusting and corrosion
- Conditions for electroplating
- The article to be electroplated is always placed at the cathode.
- The metal to be plated on the article is always made the anode and has to be replaced periodically.
- The electrolyte must contain ions of the metal which is to be plated on the article.
- A low current for a longer time should be used.
- A direct current and not alternating current should be used.
- Electroplating with Silver
Electrolyte: Na[Ag(CN)2] or K[Ag(CN)2]
Dissociation: Na[Ag(CN)2] ⇌ Na+ + Ag+ + 2CN−
H2O ⇌ H+ + OH−
Electrodes:
Cathode: Copper bell
Anode: Pure silver
Electrodes reactions:
Reaction at cathode: Ag+ + e−→ Ag (deposited)
Reaction at anode: Ag − e−→ Ag+ (goes into solution)
- Electroplating with Nickel
Electrolyte: Aqueous solution of nickel sulphate - Dissociation: NiSO4 ⇌ Ni2+ + SO42−
H2O ⇌ H+ + OH−
- Electrodes:
Cathode: Article to be electroplated
Anode: Block of pure nickel
Electrodes reactions:
Reaction at cathode: Ni2+ + 2e−→ Ni (deposited)
Reaction at anode: Ni − 2e−→ Ni2+
- Electrolytic Refining of Metals
It is a process by which metals containing impurities are purified electrolytically to give a pure metal.
Refining of Copper
Electrolyte: Copper sulphate solution and dil. sulphuric acid
Electrodes:
Cathode: Thin strip of pure copper
Anode: Impure copper
Electrode reactions:
Reaction at cathode: Cu2+ + 2e−→ Cu
Reaction at anode: Cu − 2e− → Cu2+
- Electrometallurgy
It is the process of extraction of metals by electrolysis.
Activity Series
Examples:
Extraction of Potassium
Electrolyte: Fused potassium bromide
Electrodes:
Cathode: Iron
Anode: Graphite
Reaction: KBr ⇋ K+ + Br-
Electrode reactions:
Reaction at cathode: K+ + e− ® K
Reaction at anode: Br- − e− ® Br
Br + Br ®Br2
Extraction of Magnesium
Electrolyte: Fused magnesium chloride
Electrodes:
Cathode: Iron
Anode: Graphite
Reaction: MgCl2 ⇋ Mg2+ + 2Cl−
Electrode reactions:
Reaction at cathode: Mg2+ + 2e− ® Mg
Reaction at anode: Cl− − e−® Cl
Cl + Cl ® Cl2
Download complete content for FREE 

