Metallurgy
Metallurgy Synopsis
Synopsis
- Comparison of Metals and Non-Metals
Physical Properties of Metals and Non-Metals
Chemical Properties of Metals and Non-Metals:
Occurrence of Metals
- The Earth’s crust is the major source of metals.
- Most of the metals are reactive and hence they do not occur as free elements in nature.
- Less reactive elements such as copper, silver, gold and platinum occur in a free state as metals.
- Copper and silver metals also occur in the combined state in the form of compounds.
- Mineral & Ores
Minerals: The naturally occurring compounds of metals, along with other impurities are known as minerals.
Ores: The minerals from which metals are extracted profitably and conveniently are called ores.
Gangue: Earthly impurities including silica, mud, etc. associated with the ore are called gangue.
Metallurgy: The process used for the extraction of metals in their pure form from their ores is referred to as metallurgy.
- Extraction of Metals
- The reactivity of elements differs for different metals.
- Metals at the top of the reactivity series (K, Na, Ca, Mg, Al, etc.) are reactive and they are never found in nature as free elements.
- Metals in the middle of the reactivity series (Zn, Cu, Pb, etc.) are moderately reactive.
- Metals at the bottom of the series (Au, Ag, Pt, etc.) are the least reactive and occur in a free state.
- Three major steps involved in the extraction of metals from its ore are
- Enrichment of Ores
- The ores of metal are usually contaminated with a large amount of impurities such as sand, soil, etc. called gangue.
- Before extracting the metal from an ore, it is necessary to remove these impurities.
- The method used for removing gangue from the ore depends on the differences between the physical and chemical properties of the gangue and the ore.
- Conversion of Concentrated ore into Metal
- The extraction of a metal from its concentrated ore is essentially a process of reduction of the metal compound present in the ore.
- The method of reduction to be used depends on the reactivity of the metal to be extracted.
Extraction of Less Reactive Metals
Metals at the bottom of the reactivity series are very unreactive and the oxides of these metals can be reduced by heating itself.
- Extraction of Mercury
Cinnabar, an ore of mercury is first heated in the air and is converted into mercuric oxide.
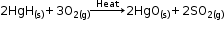
Mercuric oxide is then reduced to mercury on further heating.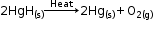
- Extraction of Copper
Concentrated copper (I) sulphide ore is roasted in the air when a part of it is oxidised to copper oxide.
When a good amount of copper (I) sulphide is converted into copper oxide, the supply of oxygen is stopped. The copper (I) oxide formed above reacts with the remaining copper (I) sulphide to form copper metal and sulphur dioxide.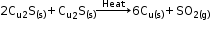
Extraction of Moderately Reactive Metals
- The moderately reactive metals in the middle of the reactivity series are extracted by the reduction of their oxides with carbon, aluminium, sodium or calcium.
- It is easier to obtain metals from their oxides (by reduction) than from carbonates or sulphides. So, before reduction can be done, the ore is converted into a metal oxide.
- The concentrated ores can be converted into metal oxides by the process of calcination or roasting.
- Calcination is the process in which a carbonate ore is heated strongly in the absence of air to convert it into a metal oxide.
For example:
When zinc carbonate is heated strongly in the absence of air, it decomposes to form zinc oxide and carbon dioxide.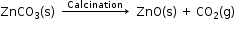
- Roasting is the process in which a sulphide ore is strongly heated in the presence of air to convert it into a metal oxide.

- The metal oxides are converted to free metal by using reducing agents such as carbon, aluminium, sodium or calcium.
For example:
- Zinc metal is extracted by the reduction of zinc oxide with carbon. Thus, when zinc oxide is heated with carbon, zinc metal is produced.
ZnO(s) + C(s) → Zn(s) + CO(g)
Manganese metal is extracted by the reduction of MnO2 with aluminium powder as the reducing agent.
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(s) + Heat - Aluminium reduces iron oxide to produce iron metal with the evolution of heat. Due to this heat, the iron metal is produced in the molten state.
- Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
The reaction of iron (III) oxide with aluminium is used to join the railway tracks or cracked machine parts. This reaction is known as the thermite reaction.
Extraction of Highly Reactive Metals
- Metals high up in the reactivity series are very reactive.
- These metals have a strong affinity for oxygen. So, oxides of sodium, magnesium, calcium and aluminium cannot be reduced by carbon.
- These metals are obtained by electrolytic reduction.
- Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
- For example:
- Sodium metal is extracted by the electrolytic reduction of molten sodium chloride.
2NaCl(l) 2Na(s) + Cl2(g)
At Cathode : Na+ + e- → Na
At Anode : 2Cl- → Cl2 + 2e- - Aluminium is extracted by the electrolytic reduction of molten aluminium oxide.
2Al2O3(l) 4Al(s) + 3O2(g)
At Cathode : Al+3 + 3e- → Al
At Anode : 2O2- → O2 + 4e-
- Refining of Metals
- The metals produced by reduction processes are not very pure and still contain impurities which must be removed to obtain pure metals.
- The most widely used method for refining impure metals is electrolytic refining.
- Electrolytic refining means refining by electrolysis. Metals such as copper, zinc, tin, lead, chromium, nickel, silver and gold are refined electrolytically.
- For refining of an impure metal by electrolysis
- A thick block of impure metal is made anode.
- A thin strip of pure metal is made cathode.
- A water soluble salt is taken as an electrolyte.
- On passing current through the electrolyte, the impure metal from the anode dissolves into the electrolyte.
- An equivalent amount of pure metal from the electrolyte is deposited on the cathode.
- The soluble impurities go into the solution, whereas the insoluble impurities settle down at the bottom of the anode and are known as the ‘anode mud’.
Metallurgy of Aluminium
- Common Ores of Aluminium
- Extraction of Aluminium
Process for Extraction of Aluminium from Bauxite
Concentration of Ore - Bayer process
- Conversion of impure bauxite to sodium aluminate
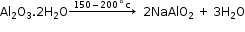
- Conversion of sodium aluminate to aluminium hydroxide
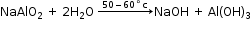
- Conversion of Al(OH)3 to pure alumina
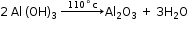
- Electrolytic Reduction in Metallic oxide – Hall Heroult process
Electrolyte
Mixture of molten alumina 20%, cryolite 60% and fluorspar 20%
Electrolytic cell
Rectangular steel tank with carbon lining
Electrodes
Cathode: Carbon lining (gas carbon)
Anode: Thick carbon (graphite)
Temperature: 950°C
Current: 100 amperes at 6–7 volts
Electrolytic reaction:
Cryolite: Na3AlF6 ⇋ 3Na1+ + Al3+ +6F1−
Fluorspar: CaF2 ⇋ Ca2+ + 2F1−
Alumina: Al2O3 ⇋ 2Al3+ + 3O2−
Cathode: 2Al3+ + 6e− → 2Al
Anode: 3O2− − 6e− → 3[O] →3O2
Anode is oxidised to carbon monoxide which further forms carbon dioxide.
2C + O2 → 2CO
2CO + O2 → 2CO2
The anode is replaced from time to time because it gets oxidised by oxygen evolved at the anode.
Products formed:
At Cathode: Pure aluminium metal
At Anode: Oxygen gas
Functions of cryolite, fluorspar and coke
Cryolite lowers the fusion temperature from 2050oC to 950oC and enhances conductivity.
Fluorspar and cryolite act as a solvent for the electrolytic mixture and increase conductivity.
Coke reduces heat loss by radiation and prevents the burning of the anode.
Refining of Aluminium (Hoope’s electrolytic process)
Tank contains three immiscible layers
Upper layer: Pure molten Al with carbon electrodes serves as the cathode.
Middle layer: Mixture of cryolite, BaF2, AlF and CaF2 serves as the electrolyte.
Lower layer: Impure Al at the bottom along with carbon lining acts as the anode.
Electrolytic reaction
Cathode: Al3+ + 3e− → Al
Anode: Al – 3e− →Al3+
Collection: Pure Al [about 99.9% pure] is withdrawn from the tapping hole.
Uses of Aluminium
- Being a strong, light and corrosion-resistant metal, it is used in alloys.
- Being a good conductor of electricity, it is used in the manufacture of cables for power transmission.
- Ships are made of alloys of aluminium because it is unaffected by sea water.
- Since it is a good reducing agent, it is used in aluminothermy. This process is used in joining broken iron girders, rails and machine parts.
Goldschmidt’s aluminothermic process (Thermite welding):
In this process, thermite (a mixture of three parts of ferric oxide and one part of aluminium powder) is taken in a crucible with a hole at the bottom.
An ignition mixture of potassium chlorate and magnesium powder is placed on the top of the thermite. To start the process, a fuse of burning magnesium is placed in the mixture which catches fire and ignites the thermite.
During the reaction, a temperature of 3000oC is achieved.
Aluminium is a powerful reducing agent. When a mixture of aluminium powder and iron oxide is ignited, the latter is reduced to metal. This process is called aluminothermy.
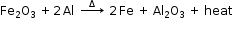
Metallurgy of Zinc
- Common Ores of Zinc
- Extraction of Zinc
- The moderately reactive metals in the middle of the reactivity series are extracted by the reduction of their oxides with carbon, aluminium, sodium or calcium.
- It is easier to obtain metals from their oxides (by reduction) than from carbonates or sulphides. So before reduction, the ore is converted to metal oxide.
- Concentrated ores can be converted to metal oxide by calcination or roasting.
- Calcination: It is the process in which a carbonate ore is heated strongly in the absence of air to convert it to a metal oxide.
Example:
When zinc carbonate is heated strongly in the absence of air, it decomposes to form zinc oxide and carbon dioxide.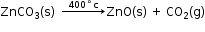
- Roasting: It is the process in which a sulphide ore is strongly heated in the presence of air to convert it to a metal oxide.
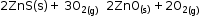
- Metal oxides are converted to free metal by using reducing agents such as carbon, aluminium, sodium or calcium.
Example:
- Zinc metal is extracted by the reduction of zinc oxide with carbon. Thus, when zinc oxide is heated with carbon, zinc metal is produced.
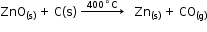
Uses of Zinc
- Mostly used for coating iron and steel sheets to prevent them from rusting.
- For making useful alloys such as brass, bronze and German silver.
- Zinc dust is used as a reducing agent in many organic reactions.
- Zinc compounds are used in paints, preservatives for leather and a mordant for the dyeing of textiles.
Metallurgy of Iron
Iron
- Common Ores of Iron
Extraction of Iron from Haematite Ore (Fe2O3)
- Concentration by gravity separation
Powdered ore is washed with a stream of water. As a result, the lighter sand particles and other impurities are washed away and heavier ore particles settle down. - Roasting and calcination
Concentrated ore is strongly heated in a limited supply of air in a reverberatory furnace. As a result, moisture is removed, and sulphur, arsenic and phosphorus impurities are oxidised off. - Smelting (in a blast furnace)
The charge consisting of roasted ore, coke and limestone in the ratio 8:4:1 is smelted in a blast furnace by introducing it through the cup and cone arrangement at the top.
- Lower region (combustion zone) - Temperature is 1500°C.
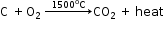
- Middle region (fusion zone) - Temperature is 1000°C.
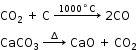
CaO + SiO2 → CaSiO3 - Upper region (reduction zone) - Temperature is 400°C.
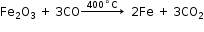
Iron thus formed is called pig iron. It is remelted and cast into different moulds. This iron is called cast iron.
Uses of Iron
- Cast iron has carbon content 2.5–5%. It is used in drain pipes, gutter covers, weights and railings.
- Wrought iron has carbon content 0.1–0.25%. It is used in chains, horse shoes and electromagnets.
- Steel has high structural and tensile strength. It is used in the construction of buildings, overhead structures, machines and in various alloys.
Important Alloys and their uses:

