Acids, Bases and Salts
Acids, Bases and Salts Synopsis
Synopsis
- Acids
An acid is a substance which dissociates (or ionises) when dissolved in water to release hydrogen ions.
For example: An aqueous solution of hydrochloric acid
HCl(aq) → H+(aq) + Cl− (aq) H+ + H2O → H3O+
The presence of hydrogen ions [H+] in hydrochloric acid solution makes it behave like an acid.
- Bases
A base is a substance which dissolves in water to produce hydroxide ions [OH- ions].
Bases which are soluble in water are called alkalis.
For example: Sodium hydroxide
NaOH(aq) → Na+(aq) + OH− (aq) - The presence of hydroxide ions [OH−] in sodium hydroxide solution makes it behave like a base.
Three important acid–base concepts
- Arrhenius Concept of Acids and Bases
According to Arrhenius theory, - Representation of the chemical equation for the ionisation of an acid HX (aq):
HX(aq) → H+(aq) + X−(aq)
or
HX(aq) + H2O(l) → H3O+(aq) + X−(aq)
The bare proton H+ is very reactive and cannot exist freely in aqueous solutions.
- Hence, it bonds to the oxygen atom of a solvent water molecule to give trigonal pyramidal hydronium ion, H3O+ {[H (H2O)]+}.
- The H+(aq) and H3O+(aq) are used interchangeably which means the same, i.e. a hydrated proton.
- Similarly, a base molecule like MOH ionises in aqueous solution according to the equation:
- MOH(aq) → M+(aq) + OH−(aq)
- The hydroxyl ion also exists in the hydrated form in the aqueous solution.
Limitation of Arrhenius concept of acid and base
- It is applicable to aqueous solutions only.
- It does not account for the basicity of substances like ammonia which do not possess a hydroxyl group.
- Brönsted–Lowry Acids and Bases
The Danish chemist Johannes Brönsted and the English chemist Thomas Lowry gave a more general definition of acids and bases.
According to Brönsted–Lowry theory,
Dissolution of ammonia (NH3) in water (H2O) can be represented by the following equation
- The basic solution is formed because of the presence of hydroxyl ions.
- In this reaction, the water molecule acts as the proton donor and the ammonia molecule acts as the proton acceptor and are thus called Brönsted–Lowry acid and base, respectively.
- n this case, NH4+ acts as a Brönsted acid, while OH− acts as a Brönsted base.
- The acid–base pair which differs only by one proton is called a conjugate acid–base pair.
- Therefore, OH− is called the conjugate base of an acid H2O and NH4+ is called the conjugate acid of the base NH3.
- If the Brönsted acid is a strong acid, then its conjugate base is a weak base and vice versa.
Ionisation of hydrochloric acid (HCl) in water (H2O) can be represented by the following equation
- HCl(aq) acts as an acid by donating a proton to the H2O molecule.
- Water acts as a base as it accepts the proton.
- The species H3O+ is produced when water accepts a proton from HCl.
- Thus, H2O is a conjugate base of an acid H3O+ and H3O+ is a conjugate acid of a base H2O.
- Similarly, Cl− is the conjugate base of HCl and HCl is the conjugate acid of the base Cl−.
- It is interesting to observe the dual role of water as an acid and a base.
- In a reaction with HCl, water acts as a base, while in a reaction with ammonia, it acts as an acid by donating a proton.
- Lewis Acids and Bases
The Lewis concept of acids and bases is a much broader concept than the previous two concepts.
According to G. N. Lewis (in 1923),
Comparisons between Brönsted–Lowry and Lewis concepts of acids and bases
A typical example based on the Lewis concept of acids and bases is the reaction of the electron-deficient species BF3 with NH3.
The reaction can be represented as follows:
BF3 + :NH3 → BF3:NH3
From the reaction, it can be observed that
- BF3 does not have a proton but still acts as an acid.
- BF3 reacts with NH3 by accepting its lone pair of electrons.
Classification of Acids
- Depending on Strength
- Strength of an Acid
The strength of an acid depends on the concentration of the hydronium ions (H3O+) present in the aqueous solution of an acid.
- Strong Acids
A strong acid vigorously ionises in aqueous solution thereby producing a high concentration of hydronium ions (H3O+).
Examples: HNO3, HCl, H2SO4
- Weak Acids
Weak acids ionise only partially in aqueous solution to produce ions and molecules.
Examples: H2CO3, CH3COOH, HCOOH
- Depending on Basicity
Basicity of an Acid
The number of hydronium ions (H3O+) which can be produced by the ionisation of one molecule of that acid in aqueous solution.
- Monobasic Acids
Acids which on ionisation in water produce one hydronium ion (H3O+) per molecule of the acid are known as monobasic acids.
Examples:
HCl + H2O ⇋ H3O+ + Cl− [Basicity = 1] - Dibasic Acids
Acids which on ionisation in water produce two hydronium ions (H3O+) per molecule of the acid are known as dibasic acids.
Examples:
H2SO4 + H2O ⇋ H3O+ + HSO4–HSO4– + H2O ⇋ H3O+ +SO4–2 [Basicity = 2] - Tribasic Acids
Acids which on ionisation in water produce three hydronium ions (H3O+) per molecule of the acid are known as tribasic acids.
Examples: - H3PO4 + H2O ⇋ H3O+ + H2PO4–
- H2PO4– + H2O ⇋ H3O+ + HPO4–2
- HPO4–2 ⇋ H3O+ + PO4–3 [Basicity = 3]
- Depending on Concentration
The concentration of an acid means the amount of acid present in a definite amount of its aqueous solution.
- Concentrated Acid
An acid which contains a very small amount of water or no water is called a concentrated acid.
- Dilute Acids
An acid which contains far more amount of water than its own mass is known as a dilute acid.
Depending on Molecular Composition
- Hydracids
Acids which contain hydrogen, a non-metallic element and no oxygen are called hydracids.
Examples: HCl, H2S, HBr, HI
- Oxyacids
Acids which contain oxygen, hydrogen and a non-metallic element are called oxyacids.
Examples: H2SO4, HNO3, H2CO3
- Preparation of Acids
By Synthesis
Hydrogen + Non-metal → Acid
H2 + Cl2 → 2HCl
H2 + Br2 → 2HBr
H2 + S → H2S - By the Action of Water o n Non-Metallic or Acidic Oxides
Acidic oxide + water → Acid
SO3 + H2O → H2SO4
SO2 + H2O → H2SO3
CO2 + H2O → H2CO3
N2O5 + H2O → 2HNO3
P2O5 + 3H2O → 2 H3PO4 -
By Oxidation of Non-Metals
Non-metal + Acid → Acid + Water + Oxide
S+ 6HNO3 → H2SO4 + 2H2O + 6NO2
P + 5HNO3 → H3PO4 + H2O + 5NO2 -
By Displacement
Salt + Less volatile acid → Salt + Acid (more volatile)
NaCl + H2SO4 → NaHSO4 + HCl
NaNO3 + H2SO4 → NaHSO4 + HNO3 -
Salt + Less volatile acid → Salt + Acid (more volatile)
NaCl + H2SO4 → NaHSO4 + HCl
NaNO3 + H2SO4 → NaHSO4 + HNO3 -
Bases
A base is either a metallic oxide or a metallic hydroxide or ammonium hydroxide which reacts with hydronium ions of an acid to form salt and water only.
Examples: CuO, NaOH, Mg(OH)2
Base + Acid → Salt + Water
CuO + 2HCl → CuCl2 + H2O
Mg(OH)2 + H2SO4 → MgSO4 + 2H2O
Al(OH)3 + 3HNO3 → Al(NO3)3 + 3H2O
NH4OH + HCl → NH4Cl + H2O.Basic Oxide
A basic oxide is a metallic oxide which contains the ion O2− and reacts with an acid to form salt and water.
Examples: NaOH, Al(OH)3 -
Alkalis
An alkali is a basic hydroxide which when dissolved in water produces hydroxyl (OH−) ions as the only negatively charged ions.
NaOH (aq) ⇋Na+ + OH–
Note: All alkalis are bases, but all bases are not alkalis.
Classification of Bases
- On the Basis of Strength
- Strong Base
It undergoes almost complete ionisation in aqueous solution to produce a high concentration of OH−.
Example:
NaOH (aq) ⇋ Na+(aq) + OH–(aq)
- Weak Base
It undergoes only partial ionisation in aqueous solution to produce a low concentration of OH− in solution.
Example:
NH4OH (aq) ⇋ NH+(aq) + OH–(aq)
Ca(OH)2(aq) ⇋ Ca2+(aq) + 2OH-(aq)
- On the Basis of Acidity
Acidity of a Base
The number of hydroxyl ions (OH–) which can be produced per molecule of the base in aqueous solution.
- Monoacidic Base
Bases which dissociate in aqueous solution to produce one hydroxyl ion (OH–) per molecule of the base are called monoacidic bases. Example:
NaOH (aq) ⇋ Na+ (aq) + OH–(aq) [Acidity = 1]
- Diacidic Base
Bases which dissociate in aqueous solution to produce two hydroxyl ions (OH–) per molecule of the base are called diacidic bases.
Example:
Ca(OH)2 (aq) ⇋ Ca2+ + 2OH– [Acidity = 2]
- Triacidic Base
Bases which dissociate in aqueous solution to produce three hydroxyl ions (OH–) per molecule of the base are called triacidic bases.
Example:
Al (OH)3 (aq) ⇋ Al3+ + 3OH– [Acidity = 3]
- On the Basis of Composition
Concentrated Alkali
An alkali having a relatively high percentage of alkali in its aqueous solution.
- Dilute Alkali
An alkali having a relatively low percentage of alkali in its aqueous solution. - Preparation of Bases
- From Metals
Metal + Oxygen → Base
2Mg + O2 →2MgO
4Na + O2 →2MgO
- By Action of Water or Steam on Reactive Metals
Reactive metal + Water → Base/Alkali + Hydrogen
2Na + 2H2O →2NaOH + H2
Ca + 2H2O →Ca (OH) 2 + H2
- By the Action of Water on Soluble Metallic Oxides
Metal oxide + Water → Base (Alkali)
Na2O + H2O →2NaOH
CaO + H2O →Ca(OH)2
- By Double Decomposition
Salt solution + Base (Alkali) → Basic hydroxide + Normal salt
FeCl3 + 3NaOH →Fe (OH) 3 + 3NaCl
CuSO4 + 2NaOH →Cu (OH) 2 + Na2SO4
AlCl3 + 3NH4OH → Al(OH)3 + 3NH4Cl
- By the Action of Oxygen on Metal Sulphides
Metallic sulphide + Oxygen → Metallic oxide + Sulphur dioxide
2ZnS + 3O2 → 2ZnO + 2SO2
2PbS + 2O2 → 2PbO + 2SO2
- By Decomposition of Salts
- Metal carbonates:
Metal carbonate →Basic oxide + Carbon dioxide
CaCO3 CaO + CO2↑
CuCO3 CuO + CO2
Sodium and potassium carbonates do not decompose on heating. - Metal nitrates:
Metal nitrate → Basic oxide + Nitrogen dioxide + Oygen
2Ca(NO3)2 2CaO + 4NO2 + O2
2Zn(NO3)2 2ZnO + 4NO2 + O2
Sodium and potassium nitrates do not give metal oxide when decomposed.
- Ammonium hydroxide can be prepared by dissolving ammonia gas in water. Ammonia is extremely soluble in water.
NH3 + H2O →NH4OH (a weak alkali)
Properties of Acids
- Physical Properties
- Acids are sour in taste in aqueous solution.
- They turn blue litmus red.
- Some acids are solids and some are liquids at room temperature.
- All strong mineral acids have corrosive action on the skin and cause painful burns.
- They are electrolytes, i.e. they conduct electricity in the aqueous state.
- Chemical Properties
Reaction with active metals
Active metal + Acid → Salt + Hydrogen
Mg + 2HCl → MgCl2 + H2↑
Zn + 2HCl → ZnCl2 + H2 ↑
Fe + H2SO4 → FeSO4 + H2 ↑
- Reaction with bases – Neutralisation
Base + Acid → Salt + Water
CuO + H2SO4 → CuSO4 + H2O
NaOH + NaNO3 → NaNO3 + H2O
- Reaction with carbonates and bicarbonates
Carbonate/bicarbonate + Acid → Salt + Water + Carbon dioxide
CaCO3 + 2HCl → CaCl2+ H2O + CO2
Ca(HCO3)2 + 2HCl →CaCl2 + 2H2O + 2CO2
- Reaction with sulphites and bisulphites
Sulphite/bisulphate + Acid → Salt + Water + Sulphur dioxide
CaSO3 + 2HCl → CaCl2 + H2O + SO2
NaHSO3 + HCl → NaCl + H2O + SO2
- Reaction with sulphides
Metal sulphide + Acid → Salt + Hydrogen sulphide
ZnS + 2HCl → ZnCl2 + H2S
FeS + H2SO4 → FeSO4 + H2S
- Reaction with chlorides:
Chlorides do not react with any of the dilute acids.
In general, they react with warm concentrated H2SO4.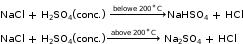
- Reaction with nitrates:
Nitrates do not react with dilute acids. However, lead nitrate reacts with both dil. HCl and dil. H2SO4.
Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3
Properties of Bases
Physical properties
- They have sharp and bitter taste.
- They change red litmus blue.
- Soapy substances, i.e. they are slippery to touch.
- They are strong electrolytes.
- They show mild corrosive action on the skin.
- Chemical properties
- Reaction with carbon dioxide:
Strong alkali + Carbon dioxide → Carbonate + Water
2NaOH + CO2 → Na2CO3 + H2O
2KOH + CO2 → K2CO3 + H2O
- Reaction with Acids – Neutralisation
Base/Alkali + Acid → Salt + Water
Ca(OH) 2 + 2HCl → CaCl2 +2H2O
Fe(OH) 2 + 2HCl → FeCl2 +2H2O
- Reaction with Metallic saltsMetallic salt + Base/Alkali →Salt + Insoluble hydroxide
CuSO4 + 2NH4OH → (NH4)2SO4 + Cu(OH) 2 ↓(pale blue)
FeSO4 + 2NaOH → Na2SO4 + Fe(OH)2 ↓(gelatinous white)
ZnSO4 + 2NaOH → Na2SO4 + Zn(OH)2↓ (dirty green)
- Reaction with ammonium salt
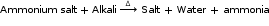
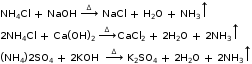
- General uses of some acids and bases:
Uses of Some Bases
Neutralisation
The reaction between an acid and a base to form salt and water is called a neutralisation reaction.
For example:
Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water.
HCl + NaOH → NaCl + H2O
pH Scale
pH value: It represents the strength of acids and alkalis expressed in terms of hydrogen ion concentration.
pH of a solution: pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in mole per litre.
pH = –log10 (H+)
pH scale: It is a scale showing the relative strength of acids and alkalis.
The normal pH scale ranges from 0 to 14 as shown below.
Indicators
They are complex substances which acquire separate colours in acidic and basic media.
Types of Indicators
Acid–Base Indicators
Common acid–base indicators such as litmus, methyl orange and phenolphthalein can distinguish between acid and basic solutions, but they cannot determine the strength of the solution.
Universal Indicator
It is a mixture of several indicators which shows different colours at different concentration of hydrogen ions in a solution.
For example:
- A universal indicator produces green colour in a neutral solution when pH = 7.
- It changes in a basic solution progressively from blue to violet as pH increases from 7 to 14.
- The colour changes from yellow to pink and then to red as pH decreases from 7 to 1.
Importance of pH in Everyday Life
a) pH change and survival of animals
- Our body works well within a narrow pH range of 7.0 to 7.8.
- When pH of rain water is less than 5.6, it is called acid rain.
- When this acid rain flows into rivers, it lowers the pH of river water and the survival of aquatic life becomes difficult.
b) pH of soil
- Most plants grow best when pH of the soil is close to 7.
- The pH of the soil can reach as low as 4 and that of basic soil can go up to 8.3.
- If the soil is too acidic, then it is treated with quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate).
- If the soil is too alkaline, then its alkalinity is reduced by adding decaying organic matter which contains acidic materials.
c) pH in our digestive system
- Our stomach produces hydrochloric acid which helps in digestion of food without harming the stomach.
- Sometimes excess acid is produced in the stomach and this causes indigestion.
- To get rid of this pain, bases called antacids are used.
- Antacids are a group of mild bases which react with excess acid and neutralise it.
- Commonly used antacids are magnesium hydroxide [Mg(OH)2] and sodium hydrogen carbonate [NaHCO3].
d) pH change - Cause of tooth decay
- Tooth decay starts when the pH of the mouth falls below 5.5.
- Tooth enamel is made up of calcium phosphate which is the hardest substance in the body.
- It is insoluble in water but gets corroded when the pH of the mouth falls below 5.5.
- Bacteria present in the mouth produce acids by the degradation of sugar and food particles after eating.
- So to prevent tooth decay, wash the mouth after eating food and brush teeth with toothpaste which is basic and neutralises excess acid.
e) Self-defense by animals and plants
Plants and animals protect themselves from enemies by injecting painful and irritating acids and bases into their skin.
Salts
A salt is a compound formed by the partial or total replacement of the ionisable hydrogen atoms of an acid by a metallic ion or an ammonium ion.
Partial Replacement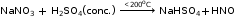
Complete Replacement
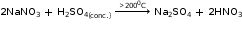
Ionic Definition of Salt
A salt is an ionic compound which dissociates in water to yield a positive ion other than the hydrogen ion (H+) and a negative ion other than the hydroxyl ion (OH−).
NaCl + H2O → Na+ + Cl−
Classification of Salts
- Normal Salts
Salts formed by the complete replacement of the replaceable hydrogen ion of an acid molecule by a basic radical.
Examples:
2NaOH + H2SO4 →Na2SO4 + 2H2SO4
HCl + NaOH →NaCl + H2O
- Acid Salt
salts formed by partial replacement of the replaceable hydrogen ion of an acid molecule by a basic radical.
Example:
NaOH + H2SO4 → NaHSO4 + H2O
Other examples: NaHSO3, Na2HPO4 and NaH2PO4
- Basic Salts
Salts formed by the partial replacement of the hydroxyl group of a di- or tri-acidic base by an acidic radical.
Example:
Mg(OH) 2 + HCl →Mg(OH)Cl + H2O
Other examples:
Basic lead chloride [Pb(OH)Cl],
Basic magnesium chloride [Mg(OH)Cl],
Basic copper chloride [Cu(OH)Cl],
Basic copper nitrate [Cu(OH)NO3]
- Doubles Salts
Salts formed by the union of two simple salts which dissolve in water and crystallise.
Examples:
Potash alum: K2SO4.Al2(SO4)3.24H2O
Mohr’s salt: FeSO4.(NH4)2SO4.6H2O
Dolomite: CaCO3.MgCO3
- Mixed Salts
Mixed salts are salts which contain more than one basic or acidic radical.
Examples:
Sodium potassium carbonate: NaKCO3
Bleaching powder: CaOCl2
- Complex Salts
Complex salts are salts which on dissociation give one simple ion and one complex ion.
Example:
Na[Ag(CN)2] ⇌Na+ + [Ag(CN)2]−
Other examples:
Silver amino chloride: [Ag(NH3)2]Cl
Tetraammine copper(II) sulphate: [Cu(NH3)4]SO4
Potassium mercuric iodide: K2[HgI4]
Sodium zincate: Na2ZnO2
General Properties of Salt
- Salts are electrovalent compounds and conduct electricity in their molten state and their aqueous solutions.
- Salts are non-volatile solids which form crystals.
- Most salts are soluble in water, and their degree of solubility varies with temperature.
- Hydrolysis of salt: The phenomenon because of which a salt formed by a weak acid and a strong base, or by a strong acid and a weak base, reacts with water to give an acidic or an alkaline solution is known as hydrolysis.
- Preparation of Soluble Salts
Preparation of Insoluble Salts
- By direct combination
Reaction:
Pb + S → PbS
Fe + S → FeS
- By combination of an acidic oxide with a basic oxide
Reaction:
SO2 + CaO → CaSO3
CO2 + CaO → CaCO3
- Double decomposition
Reactions:
- BaCl2 + H2SO4 → BaSO4 + 2HCl
- 2AgNO3 + HCl → AgCl + HNO3
- Pb(NO3)2 + Na2CO3 → PbCO3 + 2NaNO3
- Na2SO4 + BaCl2 → BaSO4 + 2NaCl
- CuSO4 + H2S → CuS + H2SO4
- Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3
- Insoluble salt can also be prepared from another insoluble salt by double decomposition.
Example:
Insoluble lead sulphate is prepared from insoluble lead oxide by converting it into soluble lead nitrate. The resulting solution is then treated with sulphuric acid resulting in the formation of white precipitate of lead sulphate.
PbO + 2HNO3 → Pb(NO3)2 + H2O
Pb(NO3)2 + H2SO4 →PbSO4 + 2NaNO3
Laboratory Preparation of Some Normal and Acid Salts
- Iron (III) chloride or anhydrous ferric chloride
It is prepared by passing dry chlorine gas over heated iron.
Fe + Cl2 → FeCl3
FeCl3 is highly deliquescent in nature, so it is kept dry in the receiver by using CaCl2.
- Copper (II) sulphate
It is prepared by the reaction of copper oxide, copper hydroxides or copper carbonates with dilute sulphuric acid.
CuO + H2SO4 → CuSO4 + H2O
Cu(OH)2 + H2SO4→ CuSO4 + 2H2O
CuCO3 + H2SO4 → CuSO4 + H2O
CuSO4 + 5H2O → CuSO4.5H2O
- Zinc sulphate and iron (II) sulphate
It is prepared by the reaction of metals with dilute sulphuric acid.
Zn + H2SO4 → ZnSO4 + H2O
ZnSO4 + 7H2O →ZnSO4.7H2O
- Lead chloride
It is prepared by adding either dilute hydrochloric acid or sodium chloride solution to a solution of lead nitrate.
Pb(NO3)2 + 2HCl ] →PbCl2 + 2HNO3
- Calcium carbonate
It is prepared by adding sodium carbonate solution to a hot solution of calcium chloride.
CaCl2 + Na2CO3 →CaCO3 + 2NaCl
- Sodium bicarbonate
- It is prepared by passing excess of carbon dioxide through a saturated solution of sodium carbonate.
Na2CO3 + CO2 + H2O → 2NaHCO3
- Sodium bicarbonate is the major constituent of baking powder.
- It is also called baking soda or sodium hydrogen carbonate.
- On a commercial scale, sodium bicarbonate is prepared by Solvay’s process.
Uses: Sodium bicarbonate is used in baking powder, as an antacid and in fire extinguishers.
- Sodium sulphate
It is prepared by drop-wise addition of dilute sulphuric acid into sodium hydroxide solution. The resulting solution contains sodium sulphate and water.
2NaOH + H2SO4 → Na2SO4 + 2H2O
- Neutralisation
It is the process by which H+ ions of an acid react completely with the [OH−] ions of a base to give salt and water only.
Example: HCl (Acid) + NaOH (Base) → NaCl (Salt) + H2O (Water)

