Organic Chemistry - I
Organic Chemistry -I Synopsis
Synopsis
Introduction
Organic Chemistry
It is the chemistry of specific carbon compounds except oxides, carbonates and carbides.
Hydrocarbons
Organic compounds composed of carbon and hydrogen only.
Examples: Methane [CH4], Ethane [C2H6]
Sources of Organic Compounds
- Plants: Compounds like sugar, starch and cellulose.
- Animals: Proteins, urea
- Coal: Destructive distillation of coal produces dyes, drugs etc.
- Petroleum: Organic compounds like gasoline, petrol and naptha are obtained from petroleum.
- Fermentation: Compounds like ethyl alcohol and acetic acid are obtained by fermentation.
- Wood: Methyl alcohol, acetone etc. are obtained by destructive distillation of wood.
- Synthetic method: Most organic compounds are synthesised in the laboratory.
Unique Nature of Carbon Atoms
- The number of carbon compounds already known at present is more than 5 million.
- Every day more new compounds are isolated or prepared in the laboratories.
- Two characteristic properties of the carbon element which lead to the formation of a very large number of organic compounds are
Tetravalency of Carbon
- Carbon forms four covalent bonds by mutually sharing its four electrons with other atoms.
- Carbon is hence tetravalent or exhibits tetravalency.
Catenation
- The property of carbon element due to which its atoms can join one another to form long carbon chains is called catenation.
- Catenation is maximum in carbon because the value of the C–C bond energy is maximum.
- Carbon undergoes self-linking forming straight, branched and closed chains.
- Straight chain of carbon atoms
- Branched chain of carbon atoms
- Closed chain or ring chain of carbon atoms
Catenation and tetravalency also result in the formation of single, double and triple bonds.
Types of Organic Compounds
Classification of Organic Compounds (Hydrocarbons)
Comparison of Saturated and Unsaturated Hydrocarbons
Isomers
Compounds with the same molecular formula but different structural formula are known as isomers, and the phenomenon is known as isomerism.
Isomers differ in physical properties or chemical properties or both.
Examples: Butane and isobutane are two different compounds with the same molecular formula C4H10.
Causes of Isomerism
- Difference in the mode of linking of atoms.
For example:C4H10O shows different types of linkages and this type of isomerism is called structural
isomerism. - Difference in the arrangement of atoms or groups in space and this type of isomerism is called stereoisomerism.
For example:
1, 2 – dichloroethene
Different types of Structure Isomerism
- Chain isomerism
Two or more compounds which have a similar molecular formula but different arrangement of carbon atoms in straight or branched chains are referred to as chain isomers, and the phenomenon is known as chain isomerism.
For example: Butane (C4H10) - Position isomerism
When two or more compounds with the same molecular formula differ in the position of the substituent atom or functional group on the carbon atom, they are called position isomers, and the phenomenon is known as position isomerism.
For example: - Functional isomerism
Two or more compounds with the same molecular formula but different functional groups are called functional isomers, and the phenomenon is known as functional isomerism.
For example:
CH3 CH2 OH and CH3 – O – CH3
Ethanol Dimethyl ether - Metamerism
It arises because of unequal distribution of alkyl groups on either side of the functional groups in the molecules.
For example:
CH3 O C3H7 and C2H5OC2H5
Methoxy propane Ethoxyethane
Homologous Series
It is a group of organic compounds with a similar structure and similar chemical properties in which the successive compounds differ by a -CH2 group.
Characteristics of a Homologous Series
- Each member of the series differs from the preceding one by the addition of a CH2 group and by 14 amu.
- All members of a homologous series share a general formula.
For example, the general formula for alkane is CnH2n+2 and that for alkene is CnH2n. - Physical properties of the members show gradation in properties as the molecular mass increases.
- The chemical properties also show gradient similarity.
For example, methane and ethane react with chlorine to form methyl chloride and ethyl chloride, respectively..
CH4 + Cl2 → CH3Cl
C2H6 + Cl2 → C2H5Cl - All members of a homologous series can be prepared by the same general method of preparation.
For example, alcohols can be prepared from alkyl halides.
CH3Br + KOH → CH3OH + KBr
C2H5Br + KOH → C2H5OH + KBr
Examples of a Homologous Series
- Alkane
- Alkene
Significance of a Homologous Series
- It helps in the systematic study of organic compounds.
- iIt helps to predict the properties and the nature of other elements of the series if the same is known of the first few members.
Nomenclature
It is the system of assigning names to organic compounds.
Systems of Nomenclature
- Trivial system
- UPAC (International Union +of Pure and Applied Chemistry) system
According to the IUPAC system, the name of an organic compound consists of three parts:
- Root word
- Suffix
- Prefix
- Root Word
It depends on the number of carbon atoms present in the longest carbon chain selected. - Suffix
- The root word is followed by an appropriate suffix which represents the nature of the bond in a carbon–carbon atom.
- Prefix
It denotes the substituent, alkyl or functional group and its position in the carbon chain.
Di-, tri- and tetra- are used for two, three and four groups of the same type, respectively.
In naming an organic compound, the following simple rules are followed:
- Selection of carbon chains: The longest continuous chain of ‘C’ atoms, known as parent chain, is selected. The longest chain need not be straight. For example:
The longest chain is of 6 carbon atoms, so the root word is ‘hex’.
(i)
(ii) - Here, the longest chain is of 7 carbon atoms, so the root word is ‘hept’, the remaining carbon atoms are substituents.
The branch chains are considered substituents, and their positions are indicated by the number of carbon atoms to which they are attached.
For example:
2-methyl (methyl is attached to the 2nd carbon) - The carbon atoms of the longest chain are numbered in such a way that the alkyl groups get the smallest possible number.
For example: - In case, any functional group is present in the chain, the carbon atoms are numbered in such a way that the functional group gets the smallest possible number.
For example: - In case, different types of substituents are attached to the chain, they are arranged and named alphabetically.
- The positions of alkyl groups are indicated by writing the position and name of the alkyl group just before the name of the parent hydrocarbon.
3-ethylheptane - Multiple alkyl groups are labelled with the Greek numerical prefixes such as ‘di’ for two, ‘tri’ for three, ‘tetra’ for four, ‘penta’ for five. If two alkyl groups are on the same carbon atom, then the numeral is repeated.
Aliphatic Hydrocarbons Alkanes:
Comparative study of Methane and Ethane:
Structure of Methane and Ethane
- Laboratory Preparation of Methane
Reactants: Sodium ethanoate and soda lime
Diagram:
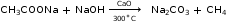
- Laboratory Preparation of Ethane
Reactants: Sodium propionate and soda lime
Reaction: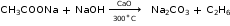
Collection: The gas is collected by the downward displacement of water.
Methods of Preparation of Methane and Ethane
- From Iodomethane or Bromoethane:
CH3I + 2[H] → CH4 + HI
C2H5Br + 2[H] → C2H6 + HBr - Methane is produced on addition of water to aluminium carbide at room temperature.
Al4C3 + 12H2O → 3CH4 ↑+ 4Al (OH)3↓ - Ethane from alkyl halides:
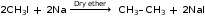
This reaction is known as the Wurtz reaction.
Properties of Methane and Ethane
- Physical Properties
Methane
- Colourless and odourless gas.
- Melting point is −183°C, and boiling point is −162°C.
- Negligibly soluble in water and soluble in organic solvents.
Ethane
- Colourless, odourless, tasteless and non-poisonous gas.
- Boiling point is −89°C, and melting point is −172°C.
- Sparingly soluble in water but soluble in organic solvents.
- Chemical Properties
- Substitution Reaction
- Reaction with halogens
Alkanes react with chlorine, bromine or iodine in the presence of sunlight or ultraviolet light to produce alkyl halides.
CH3Cl + Cl2 → CH2Cl2 + HCl
CH2Cl2 + Cl2 → CHCl3 + HCl
CHCl3 + Cl2 → CCl4 + HCl
(Carbon tetrachloride) - Reaction with oxygen (Complete combustion):
Methane and ethane burn in air with a bluish non-sooty flame to form carbon dioxide and water vapour.
CH4 + 2O2 → CO2 + 2H2O
2C2H6 + 7O2 → 4CO2 + 6H2O - Insufficient Supply of Air
Alkanes burn in an insufficient supply of air to form carbon monoxide and water.
2CH4 + 3O2 →2CO + 4H2O
2C2H6 + 5O2 →4CO + 6H2O
2. Decomposition of alkane (Cracking or Pyrolysis):
Decomposition of a compound by heat in the absence of air is called pyrolysis.
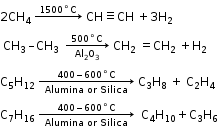
3. Catalytic Oxidation of Alkanes
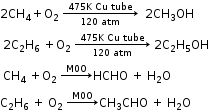
4. Slow Combustion
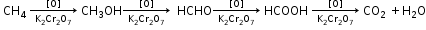
- Uses
Methane
- Methane is a source of carbon monoxide and hydrogen.
- It is used in the preparation of ethyne, methanol, methanol and chloromethane.
- It is used as a domestic fuel.
Ethane
- It is used in the preparation of ethene, ethanol and acetic acid.
- It is also a good fuel.
Alkenes:
Structure of Ethene
- Two carbon atoms linked by a double covalent bond.
- A double covalent bond is formed by sharing of two pairs of electrons between the two carbon atoms.
- Four C–H single covalent bonds and one C=C double covalent bond.
- It is a planar molecule and all bond angles (H–C–H and H–C=C) are of 120°
- Isomers in Alkenes
Alkenes with 4 or more than 4 carbon atoms can form isomers.
For example:
Butene has three isomers
- CH3 CH2 CH=CH2 (but-1-ene)
- CH3 CH=CHCH3 (but-2-ene)
- CH2=C(CH3)-CH3 (2-methyl propene)
- Preparation of Ethene
- Dehydration of ethyl alcohol
Reactants: Ethyl alcohol and conc. sulphuric acid.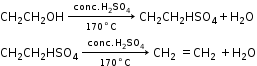
Collection: The gas is collected by the downward displacement of water because
i. It is inflammable.
ii. It is insoluble in water. - Dehydrohalogenation of ethyl bromide
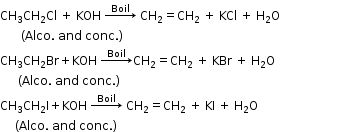
- Cracking of methane
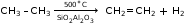
- Physical Properties
- Ethene is a colourless and inflammable gas with a pleasant odour.
- Boiling point is −102°C, and melting point is −169°C.
- Sparingly soluble in water but highly soluble in organic solvents.
- It produces an anaesthetic effect.
- Addition Reactions
- Catalytic hydrogenation
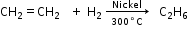
- Halogenation
- Reaction with HCl
- Addition of Water (Hydration)
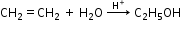
- Oxidation
- Combustion of Ethene
In excess supply of air, ethene burns with a pale blue flame to produce carbon dioxide, water and a large amount of heat.
C2H4 + 3O2 → 2CO2 + 2H2O + Heat - Polymerisation
- Uses of Ethene
- In the preparation of ethylene
- For the ripening of fruits
- For producing an oxy-ethylene flame which is used for cutting and welding of metals
- In making epoxyethane
Alkynes
- Alkynes are unsaturated aliphatic hydrocarbons containing a carbon–carbon triple bond (-C≡C-) in their molecule.
- The general formula of alkynes is CnH2n−2.
- More reactive than alkenes because of the presence of a triple bond, often referred to as an acetylenic linkage.
- Source
Acetylene is produced primarily from natural gas and from higher alkanes obtained from petroleum. - Isomerism
Alkynes with four or more than four carbon atoms can form isomers. Alkynes show position isomerism as well as chain isomerism.
For example:
Butyne shows position isomerism.
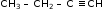
(But-1-yne)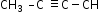
(But-2-yne)
- Structural Formula of Ethyne
- Preparation of Ethyne
- Laboratory preparation from calcium carbide
- From 1,2-dibromoethane
- From methane
- Acetylene is a colourless gas with an ether-like odour.
- It is negligibly soluble in water but soluble in organic solvents.
- It liquefies at −84°C.
- Its boiling point is −75°C.
- Addition Reactions
- Catalytic Hydrogenation
- Halogenation
Iodine reacts slowly with ethyne in the presence of alcohol to form a di-iodo derivative.
HC≡CH + I2 → ICH=CHI - Halogen Acids
HC≡CH + HBr → HCH=CHBr + HBr → CH3 – CHBr2
- Oxidation of Ethyne (Combustion)
2HC≡CH + 5O2 → 4CO2 + 2H2O + Heat
- Uses of Ethyne
- For oxy-acetylene welding at very high temperature
- As an illuminant in an oxy-acetylene lamp
- For artificial ripening and preservation of fruits
- In the manufacture of synthetic products such as polymers and artificial rubber.
- For the manufacture of important organic compounds like acetaldehyde, acetic acid, plastic and rubber
- Chemical Tests for Alkanes, Alkenes and Alkynes

