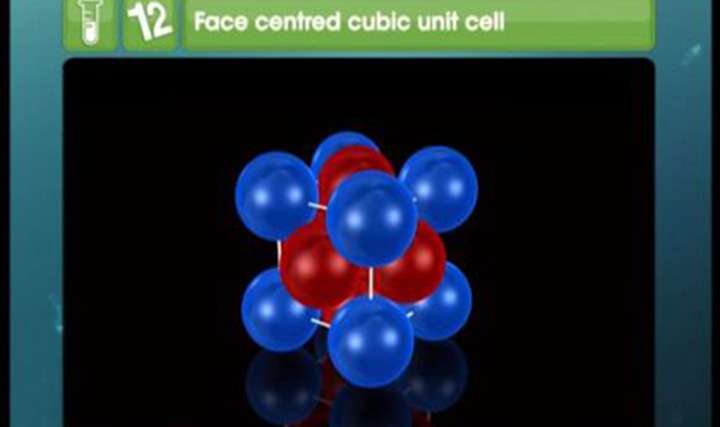
CBSE Class 12-science Chemistry Introduction to Co-ordination Compounds
Get conceptual clarity of CBSE Class 12 Science Chemistry Co-ordination Compounds – Introduction to Co-ordination Compounds with TopperLearning’s chapter resources. Learn the different types of ligands and revise the concept of coordination number with our topic notes. Understand what is a monoclinic crystal system with our concept videos.
Learning Chemistry lessons for your Class 12 Chemistry board exam can be effortless if you have adequate learning materials. On our e-learning portal, you will get CBSE Class 12 Science textbook solutions, Chemistry tests, question papers with solutions and more. With anytime access to all these resources in one place, you can prepare yourself to reach your board exam performance goals.
- how is coordination bond formed
- how is a coordinate bond formed
- The magnetic moment (in B.M.) of complex [Co(H2O)6]3+ is??
-
Solve this
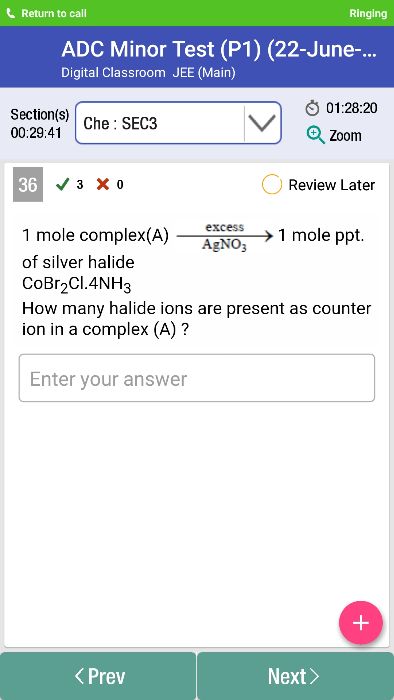
-
Pl ans

-
Explain classification of ligands on the basis of charge and dentisity. Explain the ambidentate ligands following terms with example.
Bidentate ligand
Tridentate ligands
Tetradentate ligands
Pentadentate ligands
Hexadentate ligands

- It means first we need to check what type of ligand it is then only we can state that what's its coordination number can be
- How to calculate primary valence
- Given the molecular formula of the hexa-coordinated complexes (i) CoCl3.6NH3, (ii) CoCl3.5NH3, (iii) CoCl3.4NH3. If the number of co-ordinated NH3 molecules in i, ii and iii respectively are 6, 5, 4, the primary valencies in (i), (ii) and (iii) are ?
- What is the difference between coordination entity and coordination sphere ?