CBSE Class 12-science Chemistry Crystal Field Theory
Revise CBSE Class 12 Science Chemistry Co-ordination Compounds – Crystal Field Theory with TopperLearning’s sample papers. Our sample question papers and solutions are designed by experienced teachers in line with your recent syllabus. You can use our assessment tools to gauge your understanding of the Crustal Field Theory.
Further, give yourself a disciplined approach to score top marks in your Chemistry board exam with TopperLearning’s CBSE Class 12 Science study materials. Access our online textbook solutions, solved sample papers and topic notes whenever you need them. Also, feel more confident during your board exam preparation with regular mock tests conducted using past years’ papers which are available 24/7 on our study portal.
- the degenerate orbital of[Cr(h2O)6]+3
- explain the nature of bonding in metal carbonyil
-
Please refer to the uploaded image for the doubt.
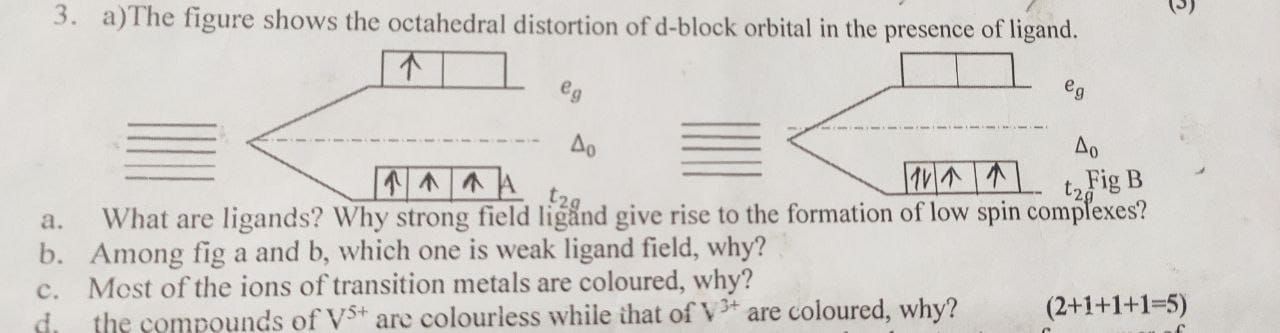
- why is [Fe(CN)6]3− a low spin complex even though it has a free electron?
- Sir pls explain crystal field theory with some example
- The structure of zeise's salt is a)octahedral b)tetrahedral c)square planar d) trigonal bipyramidal
-
Please answer the following question and also explain the structure of CuSO4.5H2O

- A. If both Assertion & Reason are True & the Reason is a correct explanation of the Assertion. B. If both Assertion & Reason are True but Reason is not a correct explanation of the Assertion. C. If Assertion is True but the Reason is False. D. If both Assertion & Reason are False. Assertion :- The dFe–O[In[Fe(H2O)6]3+] < dFe–O[In[Fe(H2O)6]2+]. Reason :- 'H2O' is a π acid ligand.
- Write the structure of Fe(CO)5.
- How the organic compounds are used in analysis?

