CBSE Class 12-science Answered
Sir pls explain crystal field theory with some example
Asked by Ajayv2021 | 22 Oct, 2019, 21:03: PM
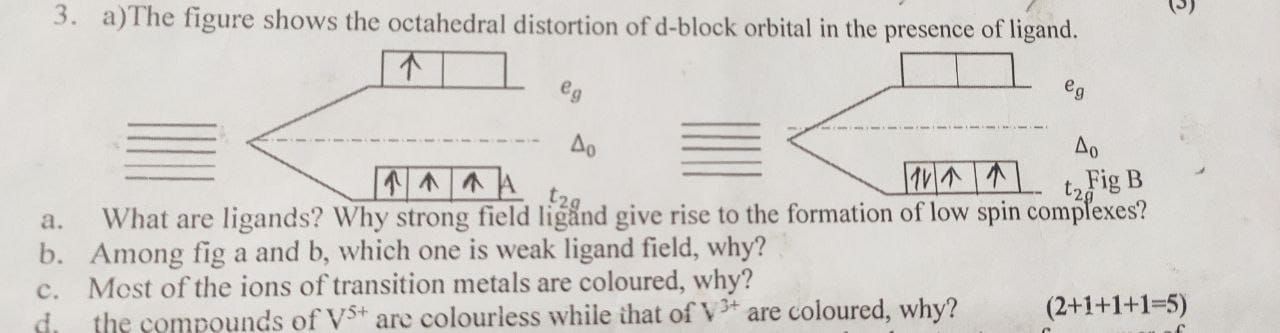
The crystal field theory considers ionic bond arising from electrostatic interaction between metal atom (positively charged) and ligands (negatively charged). The transition metal ion is considered to be positive charged equal to its oxidation state. The central metal atom is surrounded by negatively charged ligands. The ligands were considered as point charges. The energy of d orbitals remains degenerate (same energy) in the free transition metal ion. When ligand approach the metal ion, there arises attraction between the nucleus of the metal atom and the negative ends of the ligands. Due to this repulsion, the energy of the metal ion will no longer be degenerate. Consequently, the degeneracy is split into eg and t2g orbitals.
Basic concept of crystal field theory :
In transition elements d orbitals have unpaired electrons or paired electrons whenever these elements are approached by any other species like a ligand which has electron density on it, these electrons experience certain repulsion and energy of the 5 d orbitals become delocalised.
Depending on the approach of the ligand the d orbitals split themselves in order of their energy.

Basic concept of crystal field theory :
In transition elements d orbitals have unpaired electrons or paired electrons whenever these elements are approached by any other species like a ligand which has electron density on it, these electrons experience certain repulsion and energy of the 5 d orbitals become delocalised.
Depending on the approach of the ligand the d orbitals split themselves in order of their energy.
Answered by Ramandeep | 24 Oct, 2019, 15:26: PM
CBSE 12-science - Chemistry
Asked by chaudharyanu1113 | 01 Feb, 2024, 17:12: PM
CBSE 12-science - Chemistry
Asked by dabhaniamurta | 10 Jan, 2024, 07:26: AM
CBSE 12-science - Chemistry
Asked by arjunsah797 | 13 May, 2022, 18:50: PM
CBSE 12-science - Chemistry
Asked by rayyan20151 | 10 Jan, 2020, 01:23: AM
CBSE 12-science - Chemistry
Asked by Ajayv2021 | 22 Oct, 2019, 21:03: PM
CBSE 12-science - Chemistry
Asked by dongahiren88 | 12 Jul, 2019, 12:10: PM
CBSE 12-science - Chemistry
Asked by Balbir | 22 Jun, 2018, 14:07: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 18 May, 2018, 01:32: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 12:24: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM