CBSE Class 11-science Answered
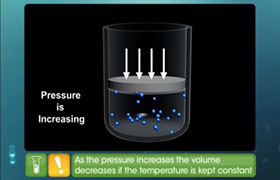
If 9.006 grams of a gas are enclosed in a 50.00 liter vessel at 273.15 K and 2.000 atmospheres of pressure, what is the molar mass of the gas? What gas is this?
Asked by Topperlearning User | 20 Apr, 2015, 11:38: AM
P = 2.000 atm
V = 50.00L
R = 0.0821 L atm mol-1 K-1
T = 273 K
We know,
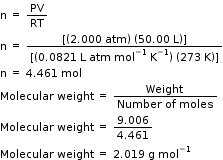
The answer (2.019 g mol-1) is approximately that of hydrogen gas, H2.
Answered by | 20 Apr, 2015, 13:38: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by pratikshyadashrkl | 12 Apr, 2020, 18:47: PM
CBSE 11-science - Chemistry
Asked by Ankit | 16 Mar, 2019, 13:01: PM
CBSE 11-science - Chemistry
Asked by minipkda | 18 Aug, 2018, 20:46: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:36: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:33: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:32: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 13:04: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:38: AM