CBSE Class 11-science Answered
at 25 degree celsius and 0.993 bar, 135 ml of a gas is collected over water. if the gas weighs 0.16g and the aqueous tension at 25 degree celsius , is 0.0317. calculate the molar mass of the gas.
Asked by Ankit | 16 Mar, 2019, 13:01: PM
Given
P = 0.993 - 0.0317
= 0.9613 bar
= 0.9487 atm
T = 25 0C
V =135 ml
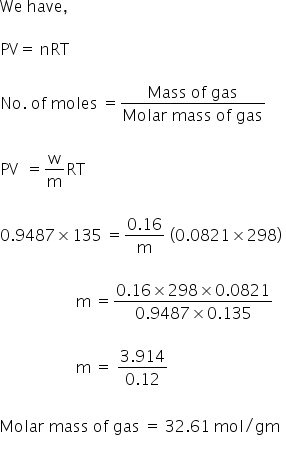
Answered by Varsha | 16 Mar, 2019, 18:51: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by pratikshyadashrkl | 12 Apr, 2020, 18:47: PM
CBSE 11-science - Chemistry
Asked by Ankit | 16 Mar, 2019, 13:01: PM
CBSE 11-science - Chemistry
Asked by minipkda | 18 Aug, 2018, 20:46: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:36: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:33: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:32: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 13:04: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:38: AM