CBSE Class 11-science Answered
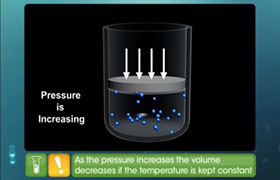
5.0g of an ideal gas occupies 9.2 L at STP. What volume would it occupy at 120°C and 92 mm Hg?
Asked by Topperlearning User | 20 Apr, 2015, 13:04: PM
PV = nRT
At STP, P = 760mm Hg, T = 273 K
P1 = 760 mm
V1 = 9.2L
T1= 273K
P1= 92 mm
V2 = ?
T2 = 120 + 273 = 393K
Using relation:

Answered by | 20 Apr, 2015, 15:04: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by pratikshyadashrkl | 12 Apr, 2020, 18:47: PM
CBSE 11-science - Chemistry
Asked by Ankit | 16 Mar, 2019, 13:01: PM
CBSE 11-science - Chemistry
Asked by minipkda | 18 Aug, 2018, 20:46: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:36: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:21: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:33: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:32: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 13:04: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 11:38: AM