CBSE Class 11-science Answered
5 moles of an idealgas expands isothermally from a pressure of 10atm to 2atm at 300k.What is the largest mass which can be lifted through a height of 1m in this expansion ? plzz explain the answer in detail
Asked by TVISHA Bhatt | 10 Aug, 2014, 06:12: PM
Solution:
Work done is given by,
w= -nRT ln (p1/p2)
= -5 mol (8.314 JK-1 mol-1)(300K) ln(10atm/2atm)
= -20.075x103J
Let M be the mass which can be lifted through a height of 1m.
Work done in lifting the mass = Mgh= M x 9.81ms-2 x 1m
M X 9.81 X 1 X m2s-2 = 20.075 x 103J
M = 2046 kg
Answered by Vaibhav Chavan | 11 Aug, 2014, 11:54: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 03:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 12:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 06:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 01:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 02:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 02:32: PM





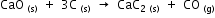 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.