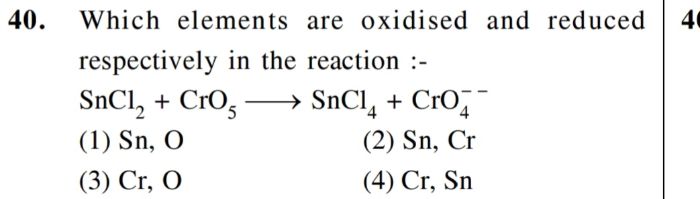CBSE Class 12-science Answered
Which transition element of the 3d series exhibit the largest number of oxidation states and why?
Asked by Topperlearning User | 26 Jun, 2014, 01:59: PM
Manganese exhibit the largest number of oxidation states. It shows the oxidation states +2, +3, +4, +5 ,+6, and + 7.The reason for that is the maximum number of unpaired electrons present in its outermost shell i.e. 3d54s2.
Answered by | 26 Jun, 2014, 03:59: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 06:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 02:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 10:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 01:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:44: PM