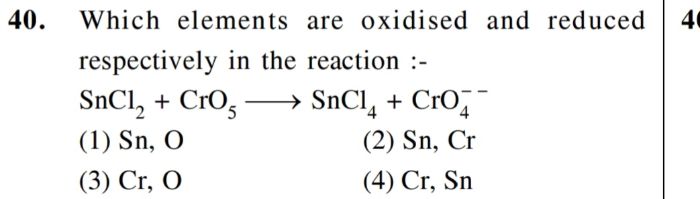CBSE Class 12-science Answered
Why zeff greater than screening effect for sc, ti,vn,cr and Mn
Asked by Debdulal | 29 Aug, 2019, 16:44: PM
In these elements outermost 4s electrons are shielded by inner 3d electrons. 3d electrons have poor shielding effect, so effective nuclear charge felt by only 4s electrons. So screening effect is lesser as compared to Z.
Answered by Ravi | 29 Aug, 2019, 19:25: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 16:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 18:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 14:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 22:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 13:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:44: PM