CBSE Class 12-science Answered
copper nitrate radical test experiment for practical with proper method to write in copy
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
Aim- To find the presence of nitrate ion in solution.
Apparatus- Test tube, beaker, Bunsen burner, Volumetric flask
Process-
Take in a test tube 1 mL of salt solution in water. Add a conc of 2 mL. Mix thoroughly with H2SO4. Cool the mixture under the tap. Add freshly prepared ferrous sulfate without shaking on the sides of the test tube. At the junction of the two solutions, a dark brown ring is formed.
Aluminium is the reducing agent in this reaction that will occur.
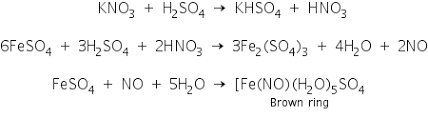
Answered by Ravi | 08 Mar, 2021, 12:22: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 16:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 18:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 14:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 22:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 13:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:44: PM