CBSE Class 9 Answered
The average atomic mass of a sample of an element X is 35.5 u. What are the percentages of isotopes 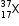 and in the sample?
and in the sample?
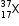 and in the sample?
and in the sample?
Asked by Topperlearning User | 04 Apr, 2016, 10:39: AM
Let the % composition of 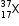 be y.
be y.
Then the % composition of 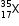 be (100 - y)
be (100 - y)
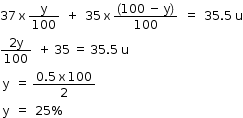
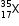 is 75% and
is 75% and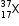 is 25% in nature.
is 25% in nature.
Answered by | 04 Apr, 2016, 12:39: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namyairmalwar9803.5sdatl | 07 Jun, 2020, 01:47: PM
CBSE 9 - Chemistry
Asked by pradoshsanesar | 10 Jan, 2020, 12:01: PM
CBSE 9 - Chemistry
Asked by maltidevi8800 | 06 Dec, 2019, 09:29: PM
CBSE 9 - Chemistry
Asked by ABHILASHA | 22 Aug, 2019, 08:46: AM
CBSE 9 - Chemistry
Asked by dubeykrishna49025 | 14 Oct, 2018, 10:02: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:05: PM









