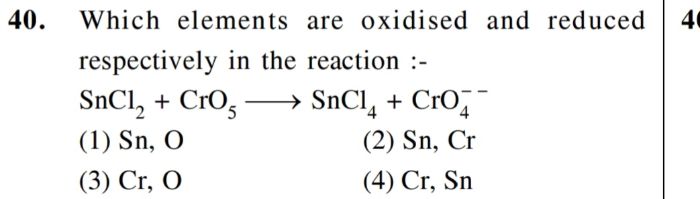CBSE Class 12-science Answered
low reduction potential of iron as well as vanadium is low. why?
Asked by ABHILASHA | 31 Jul, 2020, 07:48: AM
Vanadium will typically lose one or more electrons to form a cation, rather than gain an electron.
If it were forced to gain an electron, it probably would go to the 4s1 3d5 configuration, but the neutral atom would be a lower energy and more favorable. So, Vanadium has more tendency to oxidised than reduction so it has lower reduction potential. Iron has gereater reduction potential than vanadium but also behaves as same.
Answered by Ravi | 31 Jul, 2020, 12:18: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 06:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 02:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 10:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 01:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:44: PM