CBSE Class 12-science Answered
A
material of mass 5 g contains 3 × 1022
atoms. The nucleus of each atom possesses a charge of 25e. To give it a
charge of 4.8 μC,
what percentage of electrons must be removed from it?
Asked by Topperlearning User | 05 May, 2016, 05:40: PM
The nucleus of each atom of the material contains a charge of 25e. Therefore, each atom contains 25 electrons.
Therefore, the total number of electrons in the material is N = 25 × 3 × 1022
Now, we know that an atom as a whole is neutral. So, to give the material a net positive charge, the number of electrons to be removed is
![]()
Hence, the percentage of electrons removed is
![]()
Answered by | 05 May, 2016, 07:40: PM
Concept Videos
CBSE 12-science - Physics
Asked by niharvijayvargiya5 | 23 Apr, 2024, 06:40: PM
CBSE 12-science - Physics
Asked by manishakumariroy321 | 10 Oct, 2023, 09:02: PM
CBSE 12-science - Physics
Asked by sp6201522801 | 28 Dec, 2022, 10:09: AM
CBSE 12-science - Physics
Asked by sp6201522801 | 25 Dec, 2022, 09:33: AM
CBSE 12-science - Physics
Asked by carnivalgirl8421 | 29 Jun, 2022, 10:03: PM
CBSE 12-science - Physics
Asked by mb2022138 | 25 May, 2022, 08:37: AM
CBSE 12-science - Physics
Asked by ranjanpriy65 | 01 Apr, 2022, 12:32: PM
CBSE 12-science - Physics
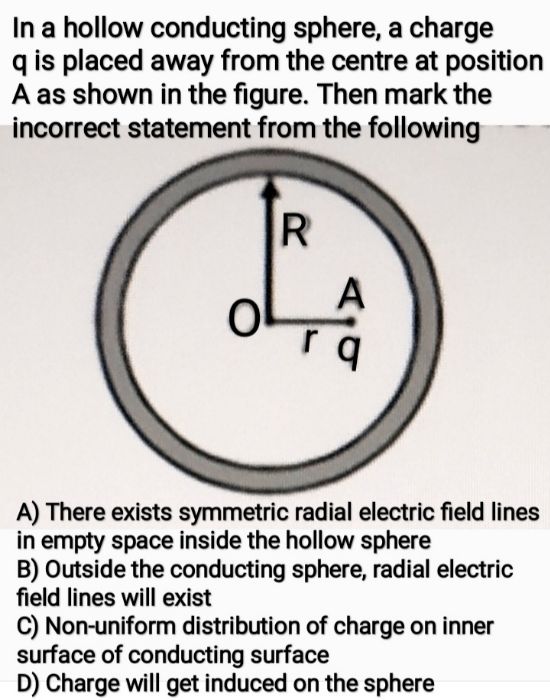
Asked by nailemwangjen79 | 14 Jul, 2021, 09:03: AM
CBSE 12-science - Physics
Asked by prakashseema091 | 31 May, 2021, 03:18: PM
CBSE 12-science - Physics
Asked by deeptykundu03 | 20 May, 2021, 12:28: PM