CBSE Class 9 Answered
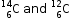 .
. (a) When the α-particles are allowed to strike on very thin gold foil, it is found that most of these particles passed through the foil without any deflection. This shows that there is a lot of empty space between the atom.
(b) The nucleus of an atom contains protons and neutrons. Protons are positively charged and neutrons are neutral. Thus, because of the presence of positively charged protons, the nucleus of an atom is positively charged.
(c) (i) Atomic number of Chlorine is 17.
Electronic configuration is 2, 8, 7.
So, it has 7 electrons in its outermost shell and it requires one electron to complete its octet.
Hence, its valency is one.
(ii) Atomic number of sulphur is 16.
Electronic configuration is 2, 8, 6.
So, it requires two electrons to complete its octet.
Hence, its valency is two.
(iii) Atomic number of magnesium is 12.
Electronic configuration is 2, 8, 2.
So, it has to give two electrons to complete its octet.
Hence, its valency is two.
OR
(a) (i) Mass numbers of isotopes of oxygen are different, i.e. 16, 17 and 18 but they have same atomic number 8.
Thus the number of electrons is same.
Therefore, they have same electronic configuration 2, 6 and same number of valence electrons.
So, their chemical properties are same.
(ii) They are electrically neutral because number of negatively charged electrons is same to the number of positively charged protons.
(b) There are three isotopes of hydrogen-
(i) Protium (ii) Deuterium (iii) Tritium
(c) One point of similarity between isotopes 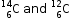 :
:
They have the same number of protons.
One point of difference between isotopes 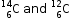 :
:
They have different number of neutrons.









