CBSE Class 9 Answered
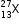 .
(i) What does the figure 13 indicate?
(ii) What does the figure 27 indicate?
(iii) What is the number of protons in the X?
(iv) What is the number of electrons in X?
OR
Give reasons for the following:
(i) Argon and calcium are called isobars.
(i) Helium and neon have zero valency.
(iii) An atom is neutral.
(iv) Nucleus is positively charged.
(v) Isotopes have similar chemical properties.
.
(i) What does the figure 13 indicate?
(ii) What does the figure 27 indicate?
(iii) What is the number of protons in the X?
(iv) What is the number of electrons in X?
OR
Give reasons for the following:
(i) Argon and calcium are called isobars.
(i) Helium and neon have zero valency.
(iii) An atom is neutral.
(iv) Nucleus is positively charged.
(v) Isotopes have similar chemical properties.(a) No, because electrons are filled first in shells having lower value of energy, the energy of first shell is lower than second shell. So, electrons will be arranged first in first shell or K shell.
(b) (i) Figure 13 indicates the atomic number of the element.
(ii) Figure 27 indicates the mass number of the element.
(iii) The number of protons in X = 13
(iv) The number of electrons in X = 13
OR
(i) Argon and calcium have the same mass number but different atomic numbers. Atoms of the elements having the same mass number but different atomic numbers are called isotopes.
(ii) Helium has two electrons in its outermost shell. Both the elements have completely filled outermost shell and cannot lose, gain or share electrons and hence their valencies are zero.
(iii) The number of positively charged protons is equal to the negatively charged electrons in an atom. thus an atom is electrically neutral.
(iv) The nucleus is made up of positively charged protons and neutral neutrons. therefore nucleus is positively charged.
9v) Isotopes are the atoms of the same elements having the same atomic number but different mass number. Since, the number of electrons or number of valence electrons in isotopes is same, hence they have similar chemical properties.









