CBSE Class 9 Answered
(a) 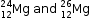 are symbols of two isotopes of Magnesium. Compare atoms of these isotopes with respect to
(i)Composition of their Nuclei
(ii)Electronic configuration and valency
(b)Give the reason why two isotopes of magnesium have different mass numbers.
are symbols of two isotopes of Magnesium. Compare atoms of these isotopes with respect to
(i)Composition of their Nuclei
(ii)Electronic configuration and valency
(b)Give the reason why two isotopes of magnesium have different mass numbers.
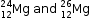 are symbols of two isotopes of Magnesium. Compare atoms of these isotopes with respect to
(i)Composition of their Nuclei
(ii)Electronic configuration and valency
(b)Give the reason why two isotopes of magnesium have different mass numbers.
are symbols of two isotopes of Magnesium. Compare atoms of these isotopes with respect to
(i)Composition of their Nuclei
(ii)Electronic configuration and valency
(b)Give the reason why two isotopes of magnesium have different mass numbers.
Asked by Topperlearning User | 04 Apr, 2016, 10:54: AM
(a)(i) 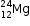
P = 12, N = Mass no. - P = 12
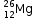
P = 12, N = 14
(ii) Electronic configuration: 2, 8, 2
Valency - 2
(b) Different mass numbers are due to presence of different number of neutrons in the two isotopes of Magnesium.
Answered by | 04 Apr, 2016, 12:54: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namyairmalwar9803.5sdatl | 07 Jun, 2020, 01:47: PM
CBSE 9 - Chemistry
Asked by pradoshsanesar | 10 Jan, 2020, 12:01: PM
CBSE 9 - Chemistry
Asked by maltidevi8800 | 06 Dec, 2019, 09:29: PM
CBSE 9 - Chemistry
Asked by ABHILASHA | 22 Aug, 2019, 08:46: AM
CBSE 9 - Chemistry
Asked by dubeykrishna49025 | 14 Oct, 2018, 10:02: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:05: PM









