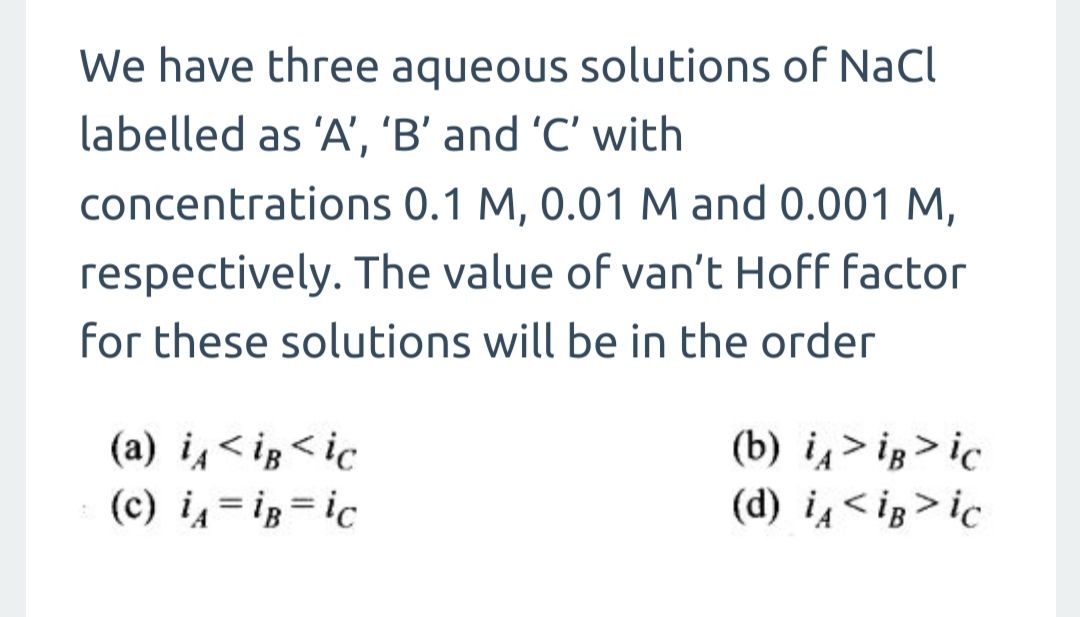
CBSE Class 12-science Answered
Two molar solution of potassium Ferro cyanide has degree of dissociation 0.70 at 300K.Find out the osmotic pressure of the solution.
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
p = i CRT
Calculation of Van't Hoff factor
K3[ Fe(CN) 6] 3 K+ + [ Fe(CN) 6]3-
1 0 0
1-α 3α α
i = 1+ 3α /1 = 1+ 3x0.70 / 1= 3.10
p= i CRT
p = 3.10 x 2 x 0.0821 x 300= 152.70 atm
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM