CBSE Class 12-science Answered
I know that answer is (a) but please explain the reason in detail
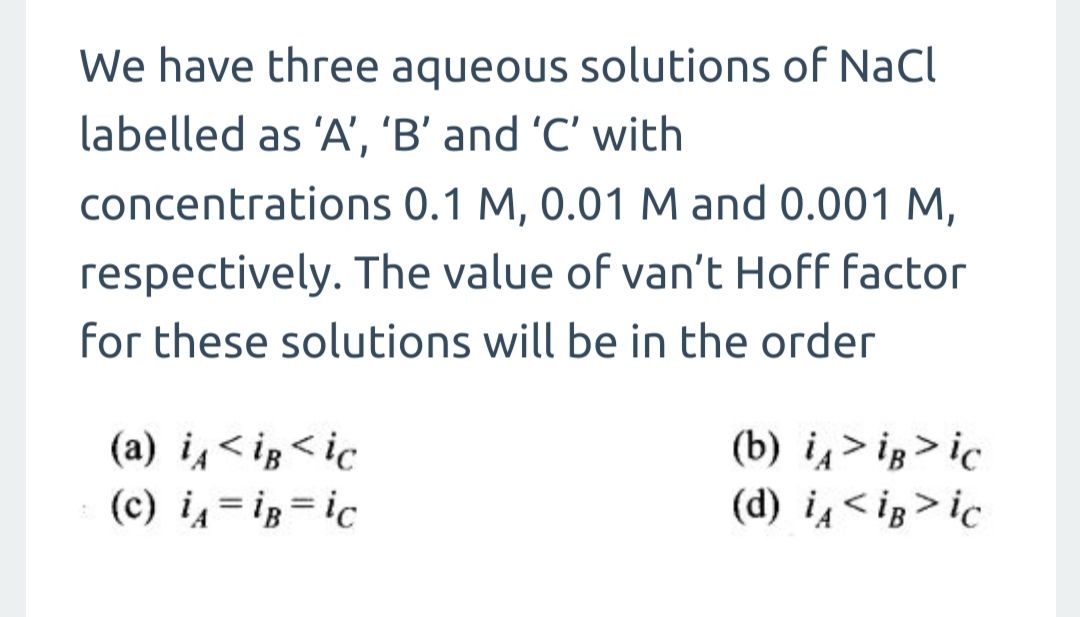
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
Van't hoff factor depennds on number of species formed after dissociation or association. It does not depend on concentration of same compound. Here number of dissociated ions is going to be same in these solutions so Van't hoff factor will remain same.
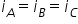
Answered by Ravi | 24 Jun, 2019, 11:43: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM





