CBSE Class 12-science - Association and Dissociation Videos
Association and dissociation of compounds
Discuss the meaning of abnormal molecular masses due to association & dissociation of compounds. Van't Hoff factor, numericals on colligative property.
More videos from this chapter
View All- The formula of degree of dissociation
- HOW TO FIND VANT OF FACTOR
-
Solution

- Value of Van't Hoff factor if CH3COOH 60% dissociates and 40% dimerized is–(1) 1.2, (2) 1.4, (3) 1.6, (4) 1.8
-
I know that answer is (a) but please explain the reason in detail
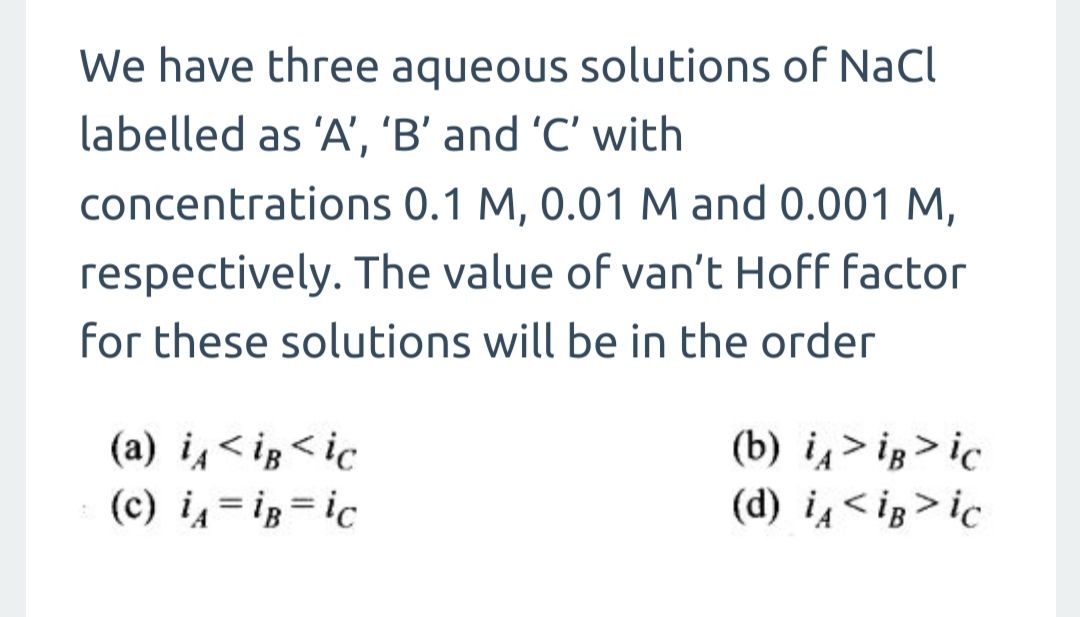
- Calculate the freezing point of a solution containing 0.52 g glucose (C6H12O6) dissolved in 80.20g water. For water Kf = 1.86 K Kg mol-1
- 0.90 g of an 1:2 electrolyte was dissolved in 87.90 g of benzene. This raised the boiling point of benzene by 0.250 C. If the molecular mass of the electrolyte is 103.0molar calculate the molal elevation constant for benzene. Given that the solute dissociate with degree of dissociation 0.25.
- 0.216molal solution of Cadmium sulphate is prepared in 1000gram of water. The depression in freezing point was measured to be 0.284K. Calculate the Vant hoff factor. The Cryoscopic constant for water is 1.86K Kg mol-1.
- Two molar solution of potassium Ferro cyanide has degree of dissociation 0.70 at 300K.Find out the osmotic pressure of the solution.
- substance X forms trimer when added to Benzene calculates the freezing point of the 0.25 molal solution. The degree of association of the solute is found to be 0.80. The freezing point of the benzene is 5.5oC and its Cryoscopic constant is 5.12 K m-1.






