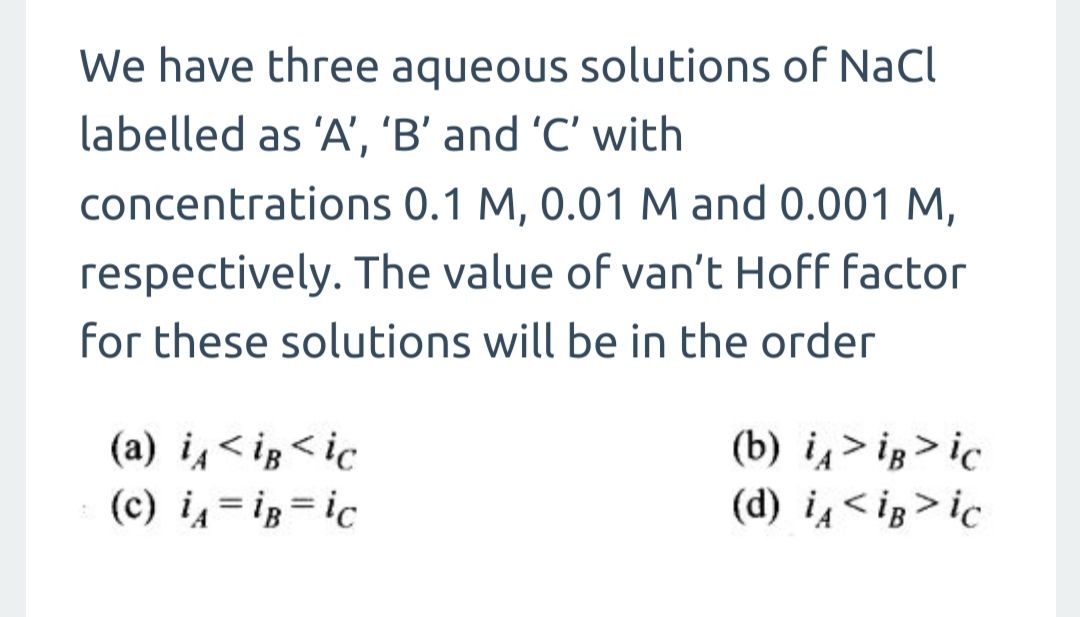
CBSE Class 12-science Answered
0.216molal solution of Cadmium sulphate is prepared in 1000gram of water. The depression in freezing point was measured to be 0.284K. Calculate the Vant hoff factor. The Cryoscopic constant for water is 1.86K Kg mol-1.
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
ΔTf = i Kf m
i = Kf m / ΔTf
I = 1.86 x 0.216 / 0.284 = 1.21
Answered by | 20 Jun, 2016, 17:50: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM