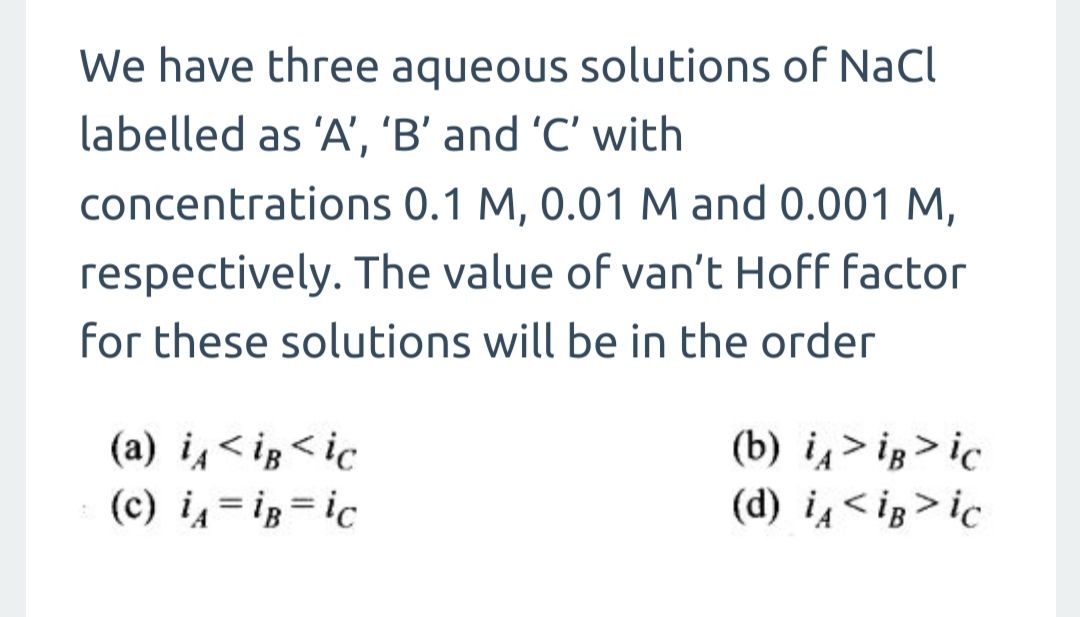
CBSE Class 12-science Answered
substance X forms trimer when added to Benzene calculates the freezing point of the 0.25 molal solution. The degree of association of the solute is found to be 0.80. The freezing point of the benzene is 5.5oC and its Cryoscopic constant is 5.12 K m-1.
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
3X X3
initial number of moles 3 0
final number of moles m (1-α) mα/3
total number of moles after association = m (1-α) + mα/3 = m (1-2α/3)
= 0.25 (1-2x0.80/3)
= 0.177m
ΔTf = Kf m
=5.12 x 0.177= 0.6
Tf (solution) = 278.65K-0.06K= 278.59K
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM