CBSE Class 12-science Answered
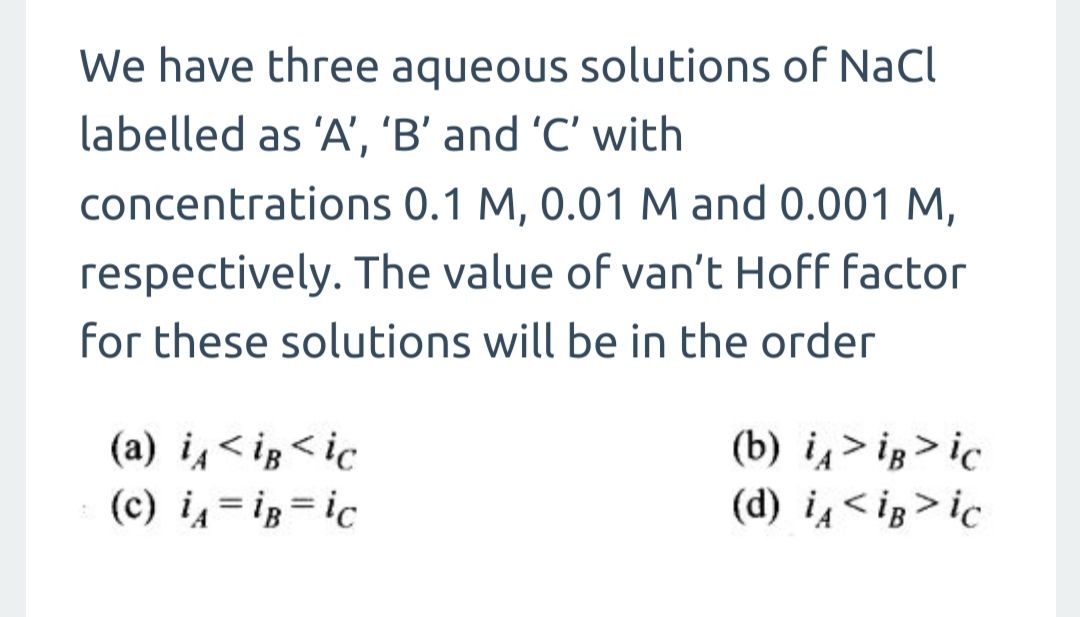
0.90 g of an 1:2 electrolyte was dissolved in 87.90 g of benzene. This raised the boiling point of benzene by 0.250 C. If the molecular mass of the electrolyte is 103.0molar calculate the molal elevation constant for benzene. Given that the solute dissociate with degree of dissociation 0.25.
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
ΔTb = i Kbm
α = i -1 / n-1
0.25 = i-1/ 3-1 i-1 = 0.50 i = 1.50
0.25 = [1.50 x Kb x 0.90/ 103] x 1000 / 87.90
= 1.50 x Kb x 0.0087 x 1000 /87.90
= 1310.67 Kb / 87.90 = 14.90
Kb = 0.25/14.90= 0.0167 K Kg mol-1
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM