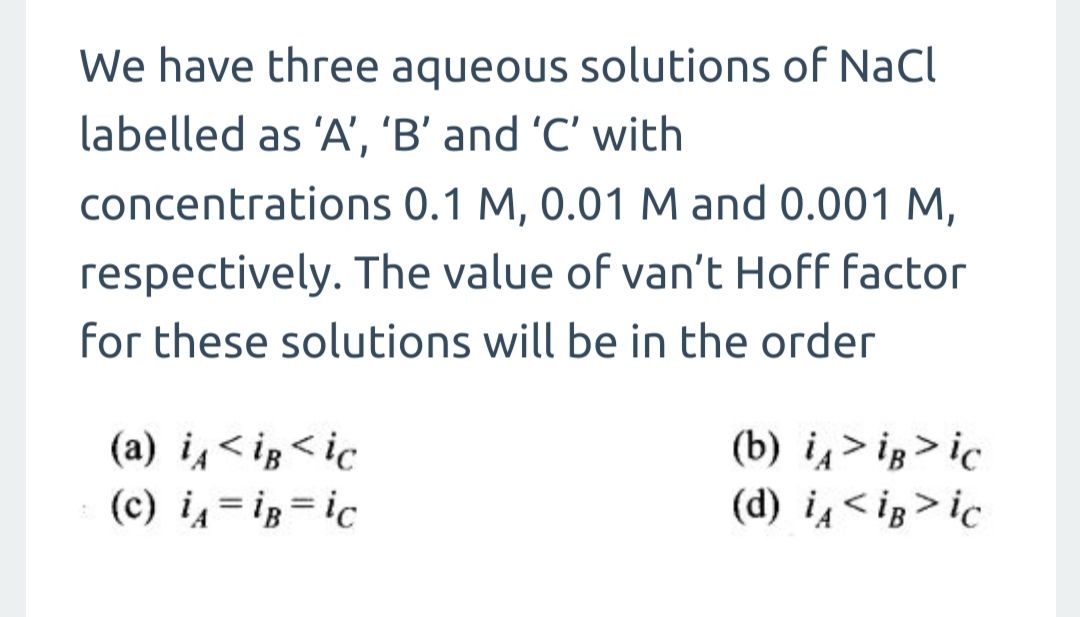
CBSE Class 12-science Answered
Calculate the freezing point of a solution containing 0.52 g glucose (C6H12O6) dissolved in 80.20g water. For water Kf = 1.86 K Kg mol-1
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
ΔTf = Kf m
ΔTf = [1.86 x (0.52/180 ) x 1000] / 80.20
ΔTf = 0.067 K
Tf (solvent) - (solution) = 0.067 K
Tf (solution) = 273.15 K-0.067 K
Tf (solution) = 273.083K
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 13:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 20:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 16:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 22:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 15:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM