CBSE Class 11-science Answered
state hess's law. how does it follow the first law of thermodynamics.
Asked by paulnaveed202 | 17 Dec, 2020, 12:36: PM
Hess's law states that theheat evolved or absorbed in a chemical process is the same whether the process takes place in one or in several steps. This is also known as the law of constant heat summation.
For Example-
Hess's law can be applied to calculate enthalpies of reactions that are difficult to measure. It is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. The application of Hess's law enables us to estimate the enthalpy of formation of CO.
C + O2 → CO2, 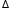 H° = -393 kJ/mol
H° = -393 kJ/mol
CO + 1/2 O2 → CO2, 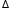 H° = -283 kJ/mol.
Subtracting the second equation from the first gives
C + 1/2 O2 → CO,
H° = -283 kJ/mol.
Subtracting the second equation from the first gives
C + 1/2 O2 → CO, 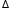 H° = -393 - (-283) = -110 kJ/mol
H° = -393 - (-283) = -110 kJ/mol
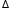 H° = -393 kJ/mol
H° = -393 kJ/molCO + 1/2 O2 → CO2,
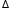 H° = -283 kJ/mol.
H° = -283 kJ/mol.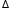 H° = -393 - (-283) = -110 kJ/mol
H° = -393 - (-283) = -110 kJ/molThe equation shows the standard enthalpy of formation of CO = -110 kJ/mol.
Answered by Ravi | 18 Dec, 2020, 18:27: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by paulnaveed202 | 17 Dec, 2020, 12:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 16:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:58: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:15: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:41: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:35: PM

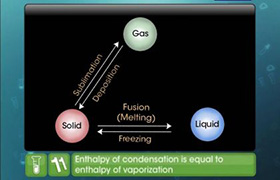


 from: Enthalpy changes of formation and bond enthalpies.
from: Enthalpy changes of formation and bond enthalpies.