CBSE Class 11-science Answered
Compare lattice Enthalpy and Ionization Enthalpy.
Asked by Topperlearning User | 14 Aug, 2014, 09:38: AM
1. There are two ways in which we can describe Lattice Energy:
The lattice dissociation enthalpy is the enthalpy change needed to convert 1 mole of solid crystal into its scattered gaseous ions. Lattice dissociation enthalpies are always positive.
The lattice formation enthalpy is the enthalpy change when 1 mole of solid crystal is formed from its scattered gaseous ions. Lattice formation enthalpies are always negative.
2. Ionisation Enthalpy is the energy required to remove the most loosely held electron from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+.
Answered by | 14 Aug, 2014, 11:38: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by paulnaveed202 | 17 Dec, 2020, 12:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 04:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:58: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:15: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:41: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM



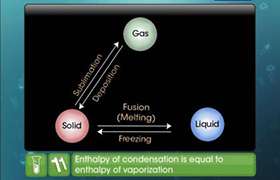
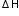 from: Enthalpy changes of formation and bond enthalpies.
from: Enthalpy changes of formation and bond enthalpies.