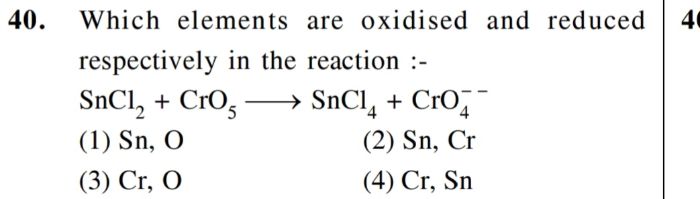CBSE Class 12-science Answered
Question

Asked by ritikabehera01 | 07 Mar, 2019, 09:28: AM
(a) From the ionisation energy point of view, we find that sum of first two ionisation energies (IE1+IE2) for Nickel is less than that of Pt, so the Ni(II) compounds will be more stable than Pt(II) compounds. Pt can also show a +4 oxidation state.
(b) Pt can form compounds of +4 oxidation state more easily as compared to Ni.
As the sum of the first four ionisation energies of Pt = IE1+IE2+IE3+IE4= 9.36 kJ/mol
(IE1+IE2+IE3+IE4)= 9.36 kJ/molis lesser than that of Ni (11.29 kJ/mol)
Answered by Ramandeep | 07 Mar, 2019, 11:36: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 16:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 18:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 14:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 22:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 13:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:44: PM