CBSE Class 11-science Answered
please explain about how the buffer solutions really work?? i mean da process wid chemical equations?
Asked by Ankana Paul | 23 Oct, 2010, 12:00: AM
Dear Student
A buffer solution is one which resists changes in pH when small quantities of an acid or an alkali are added to it.
A buffer solution has to contain things which will remove any hydrogen ions or hydroxide ions that you might add to it - otherwise the pH will change. Two categories of buffer solutions, acidic and alkaline buffer solutions achieve this in different ways.
(A) Acidic buffer solutions
An acidic buffer solution is simply one which has a pH less than 7. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt.
We'll take a mixture of ethanoic acid and sodium ethanoate. Ethanoic acid is a weak acid, and the position of this equilibrium will be well to the left:
![]()
![]()
Adding sodium ethanoate to this adds lots of extra ethanoate ions. According to Le Chatelier's Principle, that will tip the position of the equilibrium even further to the left.
(a) If another acid is added to this buffer solution most of the new hydrogen ions are removed in this way.
![]()
![]()
Since most of the new hydrogen ions are removed, the pH won't change very much - but because of the equilibria involved, it will fall a little bit.
(b) If we will add a base to this buffer solution the generated hydroxide ion is going to collide with an ethanoic acid molecule. They will react to form ethanoate ions and water.

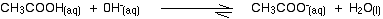
Because most of the new hydroxide ions are removed, the pH doesn't increase very much.
We hope that clarifies your query.
With Best Regards,
Team Topperlearning
We Hope that clarifies your query.
Answered by | 24 Oct, 2010, 12:41: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by gouravvv641 | 16 Aug, 2022, 09:25: PM
CBSE 11-science - Chemistry
Asked by mangalchandrj79 | 21 May, 2022, 04:38: PM
CBSE 11-science - Chemistry
Asked by sarojlaxmiacharjya | 03 Jan, 2022, 08:50: PM
CBSE 11-science - Chemistry
Asked by cjam41665 | 09 Oct, 2021, 11:11: PM
CBSE 11-science - Chemistry
Asked by rishika62124 | 03 Mar, 2021, 05:02: AM
CBSE 11-science - Chemistry
Asked by jyotijhajharia39 | 06 Jan, 2021, 11:41: PM
CBSE 11-science - Chemistry
Asked by nsaikumar33 | 15 Aug, 2020, 11:50: AM
CBSE 11-science - Chemistry
Asked by swati2678 | 10 Aug, 2020, 01:58: PM
CBSE 11-science - Chemistry
Asked by veenatripathi | 28 May, 2020, 09:03: AM










