CBSE Class 11-science Answered
in the reaction 2p +Q= 3R +S .if 2 moles each of pand Q taken initially in a 1 litre flask .at equilibrium which is true
Asked by visank90 | 24 Nov, 2023, 10:45: AM
Dear Student,
2P + Q ↔ 3R + S
Initial 2α 2α
At Equi 0 α 3α α
From the equation, 2 moles of P requires 1 mole of Q, to form 3 moles of R and 1 mole of S.
Hence, rate of consumption of P is more than rate of consumption of Q.
Therefore, at equilibrium, [P] < [Q]
Answered by | 26 Nov, 2023, 19:18: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by visank90 | 24 Nov, 2023, 10:45: AM
CBSE 11-science - Chemistry
Asked by rakhikumarithakur4 | 22 May, 2020, 20:21: PM
CBSE 11-science - Chemistry
Asked by arshrana3272 | 04 Mar, 2020, 14:57: PM
CBSE 11-science - Chemistry
Asked by hsdhall.2005 | 12 Nov, 2019, 23:40: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Jul, 2018, 20:52: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:17: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 15:08: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 16:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Apr, 2015, 16:15: PM





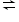 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added
6H2O (g) + 2N2 (g) How will the equilibrium shift if: a) The volume is increased, b) Helium gas is added